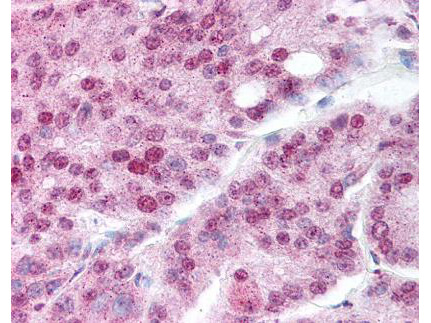
Anti-Heat Shock Protein HSP 90-alpha acetyl specific K294 (RABBIT) Antibody
HSP90 K294 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

| Host | Rabbit |
|---|---|
| Conjugate | Unconjugated |
| Target Species | Human |
| Reactivity | Human |
| Clonality | Polyclonal |
Application
| WB, IHC, E, I, LCI |
| Application Note | This affinity purified antibody has been tested for use in ELISA, immunohistochemistry and western blot. This antibody reacts strongly with the acetylated form of the immunizing peptide and shows minimal reactivity with the non-acetylated form when tested by ELISA. To date, western blotting shows equivalent staining of lysates either treated or untreated with Trichostatin A (an HDAC inhibitor). Therefore, western blotting results are not definitive for demonstrating the specificity of this reagent. Specific conditions for reactivity should be optimized by the end user. Expect a band approximately 90 kDa in size corresponding to Hsp90 protein by western blotting in the appropriate cell lysate or extract. |
| Physical State | Liquid (sterile filtered) |
| Buffer | 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 |
| Immunogen | This affinity purified antibody was prepared from whole rabbit serum produced by repeated immunizations with a synthetic peptide corresponding to amino acids surrounding K294 of human Hsp90. |
| Preservative | 0.01% (w/v) Sodium Azide |
| Gene ID | 3320 |
|---|---|
| Other Names | 3320 |
| Purity | This affinity purified antibody is directed against human Hsp90 protein acetylated at K294. The product was affinity purified from monospecific antiserum by immunoaffinity chromatography using acetyl-peptide coupled to agarose beads followed by solid phase adsorption against non-acetyl peptide. While ELISA data show strong reactivity with the acetylated form of the immunizing peptide and minimal reactivity with the non-acetylated form, to date western blotting data are not definitive for acetyl K294 specificity as blots show equivalent staining of lysates from cells either treated or untreated with Trichostatin A (an HDAC inhibitor). A BLAST analysis was used to suggest cross-reactivity with Hsp90 from human, mouse, rat, monkey, chicken and Drosophila based on 100% homology with the immunizing sequence. Reactivity of this antibody with Hsp90 from other species is unknown. |
| Storage Condition | Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use. |
| Precautions Note | This product is for research use only and is not intended for therapeutic or diagnostic applications. |
| Name | HSP90AA1 (HGNC:5253) |
|---|---|
| Synonyms | HSP90A, HSPC1, HSPCA |
| Function | Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function (PubMed:11274138, PubMed:12526792, PubMed:15577939, PubMed:15937123, PubMed:27353360, PubMed:29127155). Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself (PubMed:29127155). Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co- chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle (PubMed:26991466, PubMed:27295069). Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70 (PubMed:12526792). Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels (PubMed:25973397). In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues (PubMed:25973397). Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment (PubMed:25973397). Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression (PubMed:25973397). Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes (PubMed:11276205). Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation (PubMed:24613385). Mediates the association of TOMM70 with IRF3 or TBK1 in mitochondrial outer membrane which promotes host antiviral response (PubMed:20628368, PubMed:25609812). |
| Cellular Location | Nucleus {ECO:0000250|UniProtKB:P07901}. Cytoplasm {ECO:0000250|UniProtKB:P07901}. Melanosome. Cell membrane. Mitochondrion. Note=Identified by mass spectrometry in melanosome fractions from stage I to stage IV |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
This antibody is designed, produced, and validated as part of a collaboration between Rockland and the National Cancer Institute (NCI) and is suitable for Cancer, Immunology and Nuclear Signaling research. Hsp90 is a member of the heat shock protein 90 family, the members of which are highly conserved between isoforms and species. Hsp90 functions as a molecular chaperone and has ATPase activity. Hsp90 is a cytoplasmic protein that forms a homodimer in vivo and interacts with TOM34, AHSA1, HDAC6 and SMYD3. Several signal transduction pathways depend on Hsp90 function, including pathways involving erbB2, hypoxia sensitivity (Hif1 alpha), and steroid hormone receptors (for example, androgen, progesterone, glucocorticoid, and aryl-hydrocarbon). Recent reports show that Hsp90 from tumor cells has increased sensitivity to small molecule inhibitors (for example, 17AAG). The mechanism of the differential sensitivity of Hsp90 from normal versus tumor cells is unknown, although mutation has been ruled out. One possible mechanism may be differences in post-translational modification of tumor Hsp90. K294 was found to be acetylated in purified Hsp90 from SkBr3 cells, a breast cancer cell line.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.