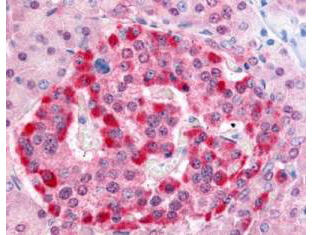
Anti-Human PARK-7 (RABBIT) Antibody
PARK-7 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

| Host | Rabbit |
|---|---|
| Conjugate | Unconjugated |
| Target Species | Human |
| Reactivity | Human |
| Clonality | Polyclonal |
Application
| WB, IHC, E, I, LCI |
| Application Note | This affinity purified antibody has been tested for use in ELISA, immunohistochemistry and western blot. Specific conditions for reactivity should be optimized by the end user. Expect a band approximately 28 kDa in size corresponding to PARK7 by western blotting in the appropriate cell lysate or extract. |
| Physical State | Liquid (sterile filtered) |
| Buffer | 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 |
| Immunogen | This affinity purified antibody was prepared from whole rabbit serum produced by repeated immunizations with a synthetic peptide corresponding to the C-terminus of Human PARK7 protein. |
| Preservative | 0.01% (w/v) Sodium Azide |
| Gene ID | 11315 |
|---|---|
| Other Names | 11315 |
| Purity | This is an affinity purified antibody produced by immunoaffinity chromatography using the immunizing peptide after immobilization to a solid phase. Reactivity occurs against human PARK7 protein. BLAST analysis was used to determine that 100% homology with the immunizing peptide sequence is on record for this protein from human, chimpanzee, African green monkey, zebrafish and also for mutant/variant forms of human DJ-1 protein. Cross reactivity with PARK7 from frog, mouse, rat, dog, chicken, Japanese rice fish and Atlantic salmon may also occur as the sequence varies by only one amino acid residue as indicated by BLAST analysis. Reactivity with PARK7 protein from other sources is not known. |
| Storage Condition | Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use. |
| Precautions Note | This product is for research use only and is not intended for therapeutic or diagnostic applications. |
| Name | PARK7 (HGNC:16369) |
|---|---|
| Function | Multifunctional protein with controversial molecular function which plays an important role in cell protection against oxidative stress and cell death acting as oxidative stress sensor and redox- sensitive chaperone and protease (PubMed:12796482, PubMed:17015834, PubMed:18711745, PubMed:19229105, PubMed:20304780, PubMed:25416785, PubMed:26995087, PubMed:28993701). It is involved in neuroprotective mechanisms like the stabilization of NFE2L2 and PINK1 proteins, male fertility as a positive regulator of androgen signaling pathway as well as cell growth and transformation through, for instance, the modulation of NF-kappa-B signaling pathway (PubMed:12612053, PubMed:14749723, PubMed:15502874, PubMed:17015834, PubMed:18711745, PubMed:21097510). Has been described as a protein and nucleotide deglycase that catalyzes the deglycation of the Maillard adducts formed between amino groups of proteins or nucleotides and reactive carbonyl groups of glyoxals (PubMed:25416785, PubMed:28596309). But this function is rebuted by other works (PubMed:27903648, PubMed:31653696). As a protein deglycase, repairs methylglyoxal- and glyoxal-glycated proteins, and releases repaired proteins and lactate or glycolate, respectively. Deglycates cysteine, arginine and lysine residues in proteins, and thus reactivates these proteins by reversing glycation by glyoxals. Acts on early glycation intermediates (hemithioacetals and aminocarbinols), preventing the formation of advanced glycation endproducts (AGE) that cause irreversible damage (PubMed:25416785, PubMed:26995087, PubMed:28013050). Also functions as a nucleotide deglycase able to repair glycated guanine in the free nucleotide pool (GTP, GDP, GMP, dGTP) and in DNA and RNA. Is thus involved in a major nucleotide repair system named guanine glycation repair (GG repair), dedicated to reversing methylglyoxal and glyoxal damage via nucleotide sanitization and direct nucleic acid repair (PubMed:28596309). Protects histones from adduction by methylglyoxal, controls the levels of methylglyoxal- derived argininine modifications on chromatin (PubMed:30150385). Able to remove the glycations and restore histone 3, histone glycation disrupts both local and global chromatin architecture by altering histone-DNA interactions as well as histone acetylation and ubiquitination levels (PubMed:30150385, PubMed:30894531). Displays a very low glyoxalase activity that may reflect its deglycase activity (PubMed:22523093, PubMed:28993701, PubMed:31653696). Eliminates hydrogen peroxide and protects cells against hydrogen peroxide-induced cell death (PubMed:16390825). Required for correct mitochondrial morphology and function as well as for autophagy of dysfunctional mitochondria (PubMed:16632486, PubMed:19229105). Plays a role in regulating expression or stability of the mitochondrial uncoupling proteins SLC25A14 and SLC25A27 in dopaminergic neurons of the substantia nigra pars compacta and attenuates the oxidative stress induced by calcium entry into the neurons via L-type channels during pacemaking (PubMed:18711745). Regulates astrocyte inflammatory responses, may modulate lipid rafts-dependent endocytosis in astrocytes and neuronal cells (PubMed:23847046). In pancreatic islets, involved in the maintenance of mitochondrial reactive oxygen species (ROS) levels and glucose homeostasis in an age- and diet dependent manner. Protects pancreatic beta cells from cell death induced by inflammatory and cytotoxic setting (By similarity). Binds to a number of mRNAs containing multiple copies of GG or CC motifs and partially inhibits their translation but dissociates following oxidative stress (PubMed:18626009). Metal-binding protein able to bind copper as well as toxic mercury ions, enhances the cell protection mechanism against induced metal toxicity (PubMed:23792957). In macrophages, interacts with the NADPH oxidase subunit NCF1 to direct NADPH oxidase-dependent ROS production, and protects against sepsis (By similarity). |
| Cellular Location | Cell membrane {ECO:0000250|UniProtKB:Q99LX0}; Lipid-anchor {ECO:0000250|UniProtKB:Q99LX0}. Cytoplasm. Nucleus. Membrane raft {ECO:0000250|UniProtKB:O88767}. Mitochondrion. Endoplasmic reticulum. Note=Under normal conditions, located predominantly in the cytoplasm and, to a lesser extent, in the nucleus and mitochondrion. Translocates to the mitochondrion and subsequently to the nucleus in response to oxidative stress and exerts an increased cytoprotective effect against oxidative damage (PubMed:18711745). Detected in tau inclusions in brains from neurodegenerative disease patients (PubMed:14705119). Membrane raft localization in astrocytes and neuronal cells requires palmitoylation |
| Tissue Location | Highly expressed in pancreas, kidney, skeletal muscle, liver, testis and heart. Detected at slightly lower levels in placenta and brain (at protein level). Detected in astrocytes, Sertoli cells, spermatogonia, spermatids and spermatozoa. Expressed by pancreatic islets at higher levels than surrounding exocrine tissues (PubMed:22611253). |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
The product of the PARK7 gene, known as PARK7, Parkinson disease (autosomal recessive, early onset) 7, DJ-1 mutant, and DJ-1 oncogene product, functions as a positive regulator of androgen receptor-dependent transcription. PARK7 may also function as a redox-sensitive chaperone and as a sensor for oxidative stress and also has been reported to prevent aggregation of SNCA. PARK7 protects neurons against oxidative stress and cell death and plays a role in fertilization. While PARK7 has no proteolytic activity, it does have a weak transforming activity. PARK7 forms a homodimer and binds to DJBP and PIAS2. PARK7 is part of a ternary complex containing PARK7, DJBP and AR and shows both a nuclear and cytoplasmic localization. Diseases include Early onset autosomal recessive Parkinson Disease 7 and Parkinson Disease Juvenile Type 2.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.