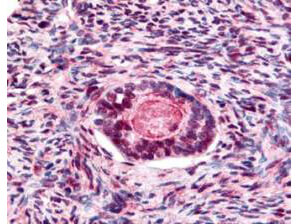
Anti-GSK3 beta pS9 (RABBIT) Antibody
GSK3 Beta phospho S9 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

| Host | Rabbit |
|---|---|
| Conjugate | Unconjugated |
| Target Species | Human |
| Reactivity | Human |
| Clonality | Polyclonal |
Application
| WB, IHC, E, I, LCI |
| Application Note | This antibody has been tested for use in ELISA, immunohistochemistry, western blotting and immunoprecipitation. Reactivity in other immunoassays is unknown. Serum starved 293T whole cell lysate is suitable for use as a positive control. Anti-GSK 3B shows a strong signal to GSK 3B in westerns at the estimated molecular weight of 47 kD. This antibody may show very weak reactivity against GSK 3A. |
| Physical State | Liquid (sterile filtered) |
| Buffer | 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 |
| Immunogen | This affinity purified antibody was prepared from whole rabbit serum produced by repeated immunizations with a synthetic peptide corresponding to a N-Terminal region near aa 1-15 of human GSK3 beta. |
| Preservative | 0.01% (w/v) Sodium Azide |
| Gene ID | 2932 |
|---|---|
| Other Names | 225903415 |
| Purity | This affinity purified antibody is directed against the phosphorylated form of human GSK3B at the pS9 residue. The product was affinity purified from monospecific antiserum by immunoaffinity purification. Antiserum was first purified against the phosphorylated form of the immunizing peptide. The resultant affinity purified antibody was then cross-adsorbed against the non-phosphorylated form of the immunizing peptide. This phospho specific polyclonal antibody reacts with phosphorylated pS9 of human GSK3B. Reactivity with non-phosphorylated human GSK3B is minimal. A BLAST analysis was used to suggest reactivity with this protein from human, rat, frog, chicken, dog and zebrafish based on 100% homology for the immunogen sequence. Cross reactivity with GSK3B homologues from other sources has not been determined. |
| Storage Condition | Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use. |
| Precautions Note | This product is for research use only and is not intended for therapeutic or diagnostic applications. |
| Name | GSK3B (HGNC:4617) |
|---|---|
| Function | Constitutively active protein kinase that acts as a negative regulator in the hormonal control of glucose homeostasis, Wnt signaling and regulation of transcription factors and microtubules, by phosphorylating and inactivating glycogen synthase (GYS1 or GYS2), EIF2B, CTNNB1/beta-catenin, APC, AXIN1, DPYSL2/CRMP2, JUN, NFATC1/NFATC, MAPT/TAU and MACF1 (PubMed:11430833, PubMed:12554650, PubMed:14690523, PubMed:16484495, PubMed:1846781, PubMed:20937854, PubMed:9072970). Requires primed phosphorylation of the majority of its substrates (PubMed:11430833, PubMed:16484495). In skeletal muscle, contributes to insulin regulation of glycogen synthesis by phosphorylating and inhibiting GYS1 activity and hence glycogen synthesis (PubMed:8397507). May also mediate the development of insulin resistance by regulating activation of transcription factors (PubMed:8397507). Regulates protein synthesis by controlling the activity of initiation factor 2B (EIF2BE/EIF2B5) in the same manner as glycogen synthase (PubMed:8397507). In Wnt signaling, GSK3B forms a multimeric complex with APC, AXIN1 and CTNNB1/beta-catenin and phosphorylates the N-terminus of CTNNB1 leading to its degradation mediated by ubiquitin/proteasomes (PubMed:12554650). Phosphorylates JUN at sites proximal to its DNA-binding domain, thereby reducing its affinity for DNA (PubMed:1846781). Phosphorylates NFATC1/NFATC on conserved serine residues promoting NFATC1/NFATC nuclear export, shutting off NFATC1/NFATC gene regulation, and thereby opposing the action of calcineurin (PubMed:9072970). Phosphorylates MAPT/TAU on 'Thr-548', decreasing significantly MAPT/TAU ability to bind and stabilize microtubules (PubMed:14690523). MAPT/TAU is the principal component of neurofibrillary tangles in Alzheimer disease (PubMed:14690523). Plays an important role in ERBB2-dependent stabilization of microtubules at the cell cortex (PubMed:20937854). Phosphorylates MACF1, inhibiting its binding to microtubules which is critical for its role in bulge stem cell migration and skin wound repair (By similarity). Probably regulates NF-kappa-B (NFKB1) at the transcriptional level and is required for the NF-kappa-B-mediated anti- apoptotic response to TNF-alpha (TNF/TNFA) (By similarity). Negatively regulates replication in pancreatic beta-cells, resulting in apoptosis, loss of beta-cells and diabetes (By similarity). Through phosphorylation of the anti-apoptotic protein MCL1, may control cell apoptosis in response to growth factors deprivation (By similarity). Phosphorylates MUC1 in breast cancer cells, decreasing the interaction of MUC1 with CTNNB1/beta-catenin (PubMed:9819408). Is necessary for the establishment of neuronal polarity and axon outgrowth (PubMed:20067585). Phosphorylates MARK2, leading to inhibition of its activity (By similarity). Phosphorylates SIK1 at 'Thr-182', leading to sustainment of its activity (PubMed:18348280). Phosphorylates ZC3HAV1 which enhances its antiviral activity (PubMed:22514281). Phosphorylates SNAI1, leading to its ubiquitination and proteasomal degradation (PubMed:15448698, PubMed:15647282, PubMed:25827072, PubMed:29059170). Phosphorylates SFPQ at 'Thr-687' upon T-cell activation (PubMed:20932480). Phosphorylates NR1D1 st 'Ser-55' and 'Ser-59' and stabilizes it by protecting it from proteasomal degradation. Regulates the circadian clock via phosphorylation of the major clock components including BMAL1, CLOCK and PER2 (PubMed:19946213, PubMed:28903391). Phosphorylates FBXL2 at 'Thr-404' and primes it for ubiquitination by the SCF(FBXO3) complex and proteasomal degradation (By similarity). Phosphorylates CLOCK AT 'Ser-427' and targets it for proteasomal degradation (PubMed:19946213). Phosphorylates BMAL1 at 'Ser-17' and 'Ser-21' and primes it for ubiquitination and proteasomal degradation (PubMed:28903391). Phosphorylates OGT at 'Ser-3' or 'Ser-4' which positively regulates its activity. Phosphorylates MYCN in neuroblastoma cells which may promote its degradation (PubMed:24391509). Regulates the circadian rhythmicity of hippocampal long-term potentiation and BMAL1 and PER2 expression (By similarity). Acts as a regulator of autophagy by mediating phosphorylation of KAT5/TIP60 under starvation conditions, activating KAT5/TIP60 acetyltransferase activity and promoting acetylation of key autophagy regulators, such as ULK1 and RUBCNL/Pacer (PubMed:30704899). Negatively regulates extrinsic apoptotic signaling pathway via death domain receptors. Promotes the formation of an anti-apoptotic complex, made of DDX3X, BRIC2 and GSK3B, at death receptors, including TNFRSF10B. The anti-apoptotic function is most effective with weak apoptotic signals and can be overcome by stronger stimulation (PubMed:18846110). Phosphorylates E2F1, promoting the interaction between E2F1 and USP11, stabilizing E2F1 and promoting its activity (PubMed:17050006, PubMed:28992046). Phosphorylates mTORC2 complex component RICTOR at 'Ser-1235' in response to endoplasmic stress, inhibiting mTORC2 (PubMed:21343617). Phosphorylates mTORC2 complex component RICTOR at 'Thr-1695' which facilitates FBXW7-mediated ubiquitination and subsequent degradation of RICTOR (PubMed:25897075). Phosphorylates FXR1, promoting FXR1 ubiquitination by the SCF(FBXO4) complex and FXR1 degradation by the proteasome (By similarity). Phosphorylates interleukin-22 receptor subunit IL22RA1, preventing its proteasomal degradation (By similarity). |
| Cellular Location | Cytoplasm. Nucleus. Cell membrane. Note=The phosphorylated form shows localization to cytoplasm and cell membrane (PubMed:20937854) The MEMO1-RHOA-DIAPH1 signaling pathway controls localization of the phosphorylated form to the cell membrane (PubMed:20937854) |
| Tissue Location | Expressed in testis, thymus, prostate and ovary and weakly expressed in lung, brain and kidney. Colocalizes with EIF2AK2/PKR and TAU in the Alzheimer disease (AD) brain |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Glycogen synthase kinase-3 (GSK 3) is a proline-directed serine-threonine kinase that was initially identified as a phosphorylating and inactivating glycogen synthase. Two isoforms, alpha (GSK 3A) and beta, show a high degree of amino acid homology. GSK 3B is involved in energy metabolism, neuronal cell development, and body pattern formation. GSK 3B participates in the Wnt signaling pathway and has been implicated in the hormonal control of several regulatory proteins including glycogen synthase, MYB and the transcription factor JUN. GSK 3B phosphorylates JUN at sites proximal to its DNA-binding domain, thereby reducing its affinity for DNA.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.











 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.