HSP90 (total) Antibody
HSP90 Antibody, Clone 4F3.E8
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

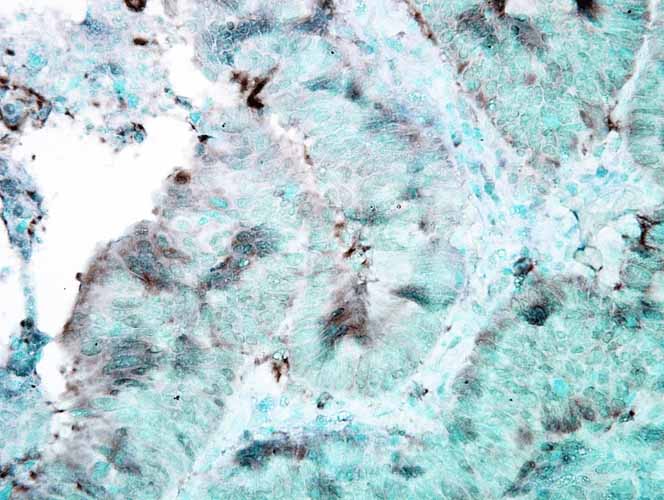
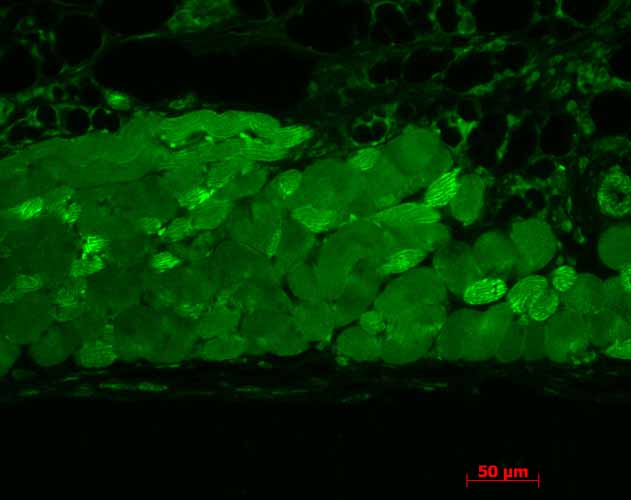
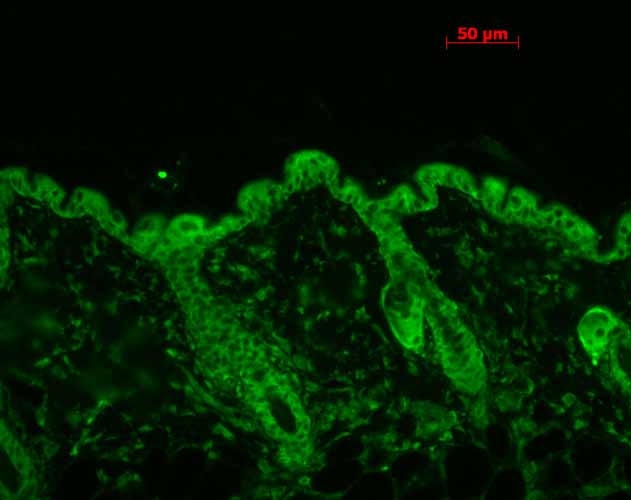
Application
| WB, IHC, ICC, IP, E |
|---|---|
| Primary Accession | P07900 |
| Other Accession | NP_001017963.2 |
| Host | Mouse |
| Isotype | IgG2a |
| Reactivity | Human, Mouse, Rat |
| Clonality | Monoclonal |
| Description | Mouse Anti-Human HSP90 (total) Monoclonal IgG2a |
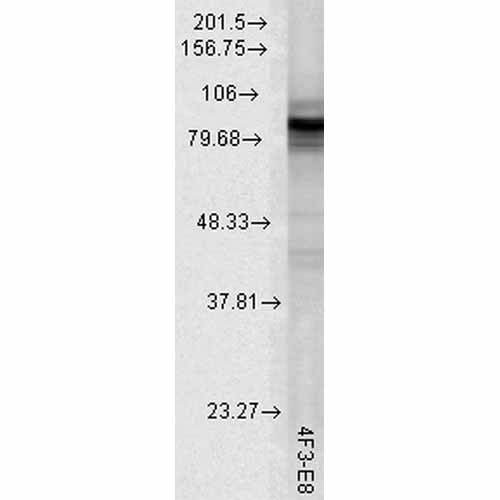
| Target/Specificity | Detects ~90kDa. This antibody detects both α and β forms of HSP90 equally well. |
| Other Names | HSP84 Antibody, HSP86 Antibody, HSP90A Antibody, HSP90AA1 Antibody, HSP90AB1 Antibody, HSP90B Antibody, HSPC1 Antibody, HSPC2 Antibody, HSPCAL1 Antibody, HSPCAL4 Antibody |
| Clone Names | 4F3.E8 |
| Immunogen | Recombinant Human HSP90 purified from E.coli |
| Purification | Protein G Purified |
| Storage | -20ºC |
| Storage Buffer | PBS pH7.2, 50% glycerol, 0.09% sodium azide |
| Shipping Temperature | Blue Ice or 4ºC |
| Certificate of Analysis | 0.5 µg/ml of SMC-149 was sufficient for detection of HSP90alpha in 20 µg of heat shocked HeLa cell lysate by colorimetric immunoblot analysis using Goat anti-mouse IgG:HRP as the secondary antibody. |
| Cellular Localization | Cytoplasm | Melanosome |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
HSP90 is an abundantly and ubiquitously expressed heat shock protein. It is understood to exist in two principal forms α and β, which share 85% sequence amino acid homology. The two isoforms of HSP90 are expressed in the cytosolic compartment (1). Despite the similarities, HSP90α exists predominantly as a homodimer while HSP90β exists mainly as a monomer (2). From a functional perspective, HSP90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex (3-6). Furthermore, HSP90 is highly conserved between species; having 60% and 78% amino acid similarity between mammalian and the corresponding yeast and Drosophila proteins, respectively. HSP90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. Despite its label of being a heat-shock protein, HSP90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the HSP90-regulated proteins that have been discovered to date are involved in cell signaling (7-8). The number of proteins now know to interact with HSP90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase (5). When bound to ATP, HSP90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, HSP90-interacting proteins have been shown to co-precipitate with HSP90 when carrying out immunoadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in HSP90 expression or HSP90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit HSP90 function (9). For more information visit our HSP90 Scientific Resource Guide at http://www.HSP90.ca.
References
1. Nemoto T. et al. (1997) J.Biol Chem. 272: 26179-26187.
2. Minami, Y, et al. (1991), J.Biol Chem. 266: 10099-10103.
3. Arlander SJH, et al. (2003) J Biol Chem 278: 52572-52577.
4. Pearl H, et al. (2001) Adv Protein Chem 59: 157-186.
5. Neckers L, et al. (2002) Trends Mol Med 8: S55-S61.
6. Pratt W, Toft D. (2003) Exp Biol Med 228: 111-133.
7. Pratt W, Toft D. (1997) Endocr Rev 18: 306–360.
8. Pratt WB. (1998) Proc Soc Exptl Biol Med 217: 420–434.
9. Whitesell L, et al. (1994) Proc Natl Acad Sci USA 91: 8324–8328.
10. Nemoto, T. (1997) Biochem and Mol. Bio Intl. 42 (5): 881-889.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.