Alpha A Crystallin Antibody
Alpha A Crystallin Antibody, Clone 1H3.B8
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, E, ICC |
|---|---|
| Primary Accession | P02489 |
| Other Accession | NP_000385.1 |
| Host | Mouse |
| Isotype | IgG1 |
| Reactivity | Human, Mouse, Rat, Bovine |
| Clonality | Monoclonal |
| Description | Mouse Anti-Human Alpha A Crystallin Monoclonal IgG1 |
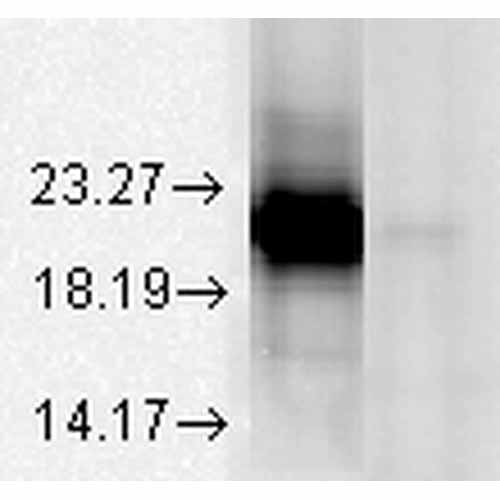
| Target/Specificity | Detects ~20kDa. Does not cross-react with αB-crystallin, βL-crystallin, ΒH- crystallin, γ-crystallin, HSP25, HSP27 or HSP47 proteins. |
| Other Names | Heat shock protein beta4 Antibody, Acry 1 Antibody, CRYA1 Antibody, CRYAA Antibody, HSPB4 Antibody |
| Clone Names | 1H3.B8 |
| Immunogen | Native Alpha Crystallin |
| Purification | Protein G Purified |
| Storage | -20ºC |
| Storage Buffer | PBS pH7.2, 50% glycerol, 0.09% sodium azide |
| Shipping Temperature | Blue Ice or 4ºC |
| Certificate of Analysis | 0.5 µg/ml was sufficient for detection of 100 ng purified alphaA crystalline by colorimetric immunoblot analysis using Goat Anti-Mouse IgG:HRP as the secondary. |
| Cellular Localization | Cytoplasm | Nucleus |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
The alpha-crystallins are major water-soluble lens structural proteins of the vertebrate eye that are related to the small heat shock protein family. The alpha-crystallins possess structural and functional similarities with HSP25 and HSP27 (1). Mammalian lens cystallins are divided into alpha, beta and gamma families. Alpha and beta families are further divided into acidic and basic groups (Alpha-A and Alpha-B respectively). In the lens, alpha-crystallin primarily functions to maintain proper refractive index, however it can also function as a molecular chaperone that binds to the denatured proteins, keeping them in solution and thereby maintaining the translucency of the lens. When cellular stress occurs, alpha-crystallin enters its’ phosphorylated state and may serve a structural control function and play a role in protein maintenance (2). In addition to their interaction with proteins, alpha-crystallins also interact with native molecules such as membrane proteins, Golgi matrix protein, structural proteins, nuclear proteins and DNA (3, 4, 5, 6, and 7). Two other functions are an autokinase activity and participation in the intracellular architecture, and it has also been proven that both alpha-A and B prevent apoptosis by inhibiting caspases (8).
References
1. Merck K.B. et al. (1993) J Biol Chem. 268: 1046-1052.
2. Horwitz J. (1992) Proc Natl Acad Sci USA 89(21): 10449-10453.
3. Cobb B.A. and Petrash J.M. (2002) Biochemistry. 41: 483-490
4. Horwitz J. (2003) Exp Eye Res. 76: 145-153.
5. Bullard B. et al. (2004) J Biol Chem. 279: 7917-7924.
6. Gangalum R.K., Schibler M.J. and Bhat S.P. (2004) J Biol Chem. 279: 43374.43377.
7. Maddala R. and Rao V.P. (2005) Exp Cell Res. 306: 203-215.
8. Yaung J., et al. (2007) Molecular Vision 13: 566-577.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.











 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.