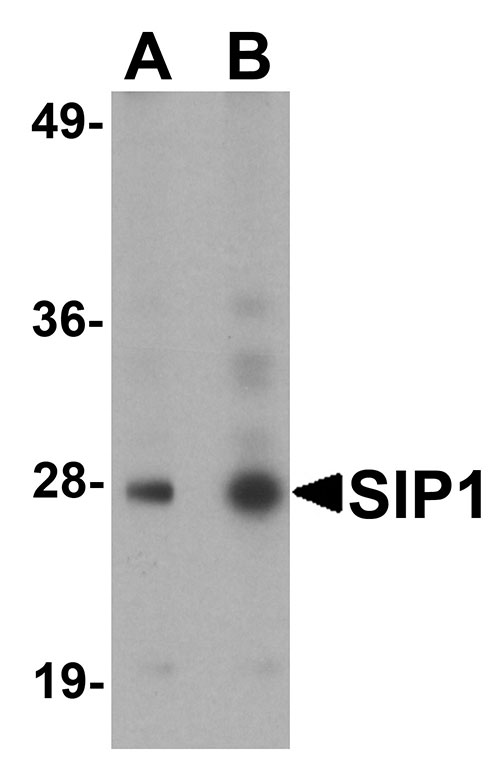
SIP1 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IF, ICC, E |
|---|---|
| Primary Accession | O14893 |
| Other Accession | AAB82297, 2570925 |
| Reactivity | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Calculated MW | 31585 Da |
| Application Notes | SIP1 antibody can be used for detection of SIP1 by Western blot at 0.5 - 1 µg/mL. Antibody can also be used for immunocytochemistry starting at 4 µg/mL. For immunofluorescence start at 20 µg/mL. |
| Gene ID | 8487 |
|---|---|
| Target/Specificity | SIP1; |
| Reconstitution & Storage | SIP1 antibody can be stored at 4℃ for three months and -20℃, stable for up to one year. As with all antibodies care should be taken to avoid repeated freeze thaw cycles. Antibodies should not be exposed to prolonged high temperatures. |
| Precautions | SIP1 Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | GEMIN2 (HGNC:10884) |
|---|---|
| Synonyms | SIP1 |
| Function | The SMN complex catalyzes the assembly of small nuclear ribonucleoproteins (snRNPs), the building blocks of the spliceosome, and thereby plays an important role in the splicing of cellular pre- mRNAs (PubMed:18984161, PubMed:9323129). Most spliceosomal snRNPs contain a common set of Sm proteins SNRPB, SNRPD1, SNRPD2, SNRPD3, SNRPE, SNRPF and SNRPG that assemble in a heptameric protein ring on the Sm site of the small nuclear RNA to form the core snRNP (Sm core) (PubMed:18984161). In the cytosol, the Sm proteins SNRPD1, SNRPD2, SNRPE, SNRPF and SNRPG (5Sm) are trapped in an inactive 6S pICln-Sm complex by the chaperone CLNS1A that controls the assembly of the core snRNP (PubMed:18984161). To assemble core snRNPs, the SMN complex accepts the trapped 5Sm proteins from CLNS1A (PubMed:18984161, PubMed:9323129). Binding of snRNA inside 5Sm ultimately triggers eviction of the SMN complex, thereby allowing binding of SNRPD3 and SNRPB to complete assembly of the core snRNP (PubMed:31799625). Within the SMN complex, GEMIN2 constrains the conformation of 5Sm, thereby promoting 5Sm binding to snRNA containing the snRNP code (a nonameric Sm site and a 3'-adjacent stem-loop), thus preventing progression of assembly until a cognate substrate is bound (PubMed:16314521, PubMed:21816274, PubMed:31799625). |
| Cellular Location | Nucleus, gem. Cytoplasm. Note=Localized in subnuclear structures next to coiled bodies, called gems, which are highly enriched in spliceosomal snRNPs. Also found in the cytoplasm |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
SIP1 Antibody: SIP1 is one of the proteins found in the SMN complex, which consists of the survival of motor neuron (SMN) protein and several gemin proteins. The SMN complex is localized to a subnuclear compartment called gems (gemini of coiled bodies) and is required for assembly of spliceosomal snRNPs and for pre-mRNA splicing. SIP1 interacts directly with the SMN and it is required for formation of the SMN complex. A knockout mouse targeting the mouse homolog of this gene exhibited disrupted snRNP assembly and motor neuron degeneration. However, knockdown of the SIP1 mRNA in motor neurons showed normal motor axons while that of SMN mRNA did show abnormal motor axon outgrowth, indicating that SIP1 may have additional roles outside of the SMN complex.
References
Liu Q, Fischer U, Wang F, et al. The spinal muscle atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell1997; 90:1013-21.
Ogawa C, Usui K, Aoki M, et al. Gemin2 plays an important role in stabilizing the survival of motor neuron complex. J. Biol. Chem.2007; 282:11122-34.
Jablonka S, Holtmann B, Meister G, et al. Gene targeting of Gemin2 in mice reveals a correlation between defects in the biogenesis of U snRNPs and motoneuron cell death. Proc. Natl. Acad. Sci. USA2002; 99:10126-31.
McWhorter ML, Boon KL, Horan ES, et al. The SMN binding protein Gemin2 is not involved in motor axon outgrowth. Dev. Neurobiol.2008; 68:182-94.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.