TEM1 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IHC-P, IF, E |
|---|---|
| Primary Accession | Q9HCU0 |
| Other Accession | NP_065137, 57124 |
| Reactivity | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
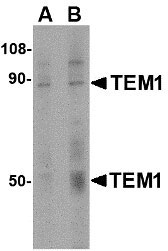
| Calculated MW | Predicted: 48, 83 kDa Observed: 50, 88 kDa |
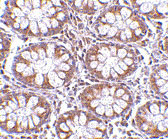
| Application Notes | TEM1 antibody can be used for detection of TEM1 by Western blot at 0.5 - 1 μg/mL. Antibody can also be used for immunohistochemistry starting at 2.5 μg/mL. For immunofluorescence start at 20 μg/mL. |
| Gene ID | 57124 |
|---|---|
| Target/Specificity | TEM1 antibody was raised against a 14 amino acid synthetic peptide near the carboxy terminus of the human TEM1. The immunogen is located within the last 50 amino acids of TEM1. |
| Reconstitution & Storage | TEM1 antibody can be stored at 4℃ for three months and -20℃, stable for up to one year. As with all antibodies care should be taken to avoid repeated freeze thaw cycles. Antibodies should not be exposed to prolonged high temperatures. |
| Precautions | TEM1 Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | CD248 |
|---|---|
| Synonyms | CD164L1, TEM1 |
| Function | Cell surface glycoprotein involved in various biological processes including angiogenesis, immune response modulation, and tissue remodeling and repair. Participates in pericyte proliferation through positive modulation of the PDGF receptor signaling pathway (PubMed:20484976). Acts as a scaffold for factor X, triggering allosteric changes and the spatial re-alignment of factor X with the TF-factor VIIa complex, thereby enhancing coagulation activation. Modulates the insulin signaling pathway by interacting with insulin receptor/INSR and by diminishing its capacity to be autophosphorylated in response to insulin. Also regulates LPS-induced inflammatory response in macrophages by favoring the production of proinflammatory cytokines. In human, negatively regulates T-cell proliferation compared with stromal cells where it increases proliferation (PubMed:21466550). |
| Cellular Location | Membrane; Single-pass type I membrane protein |
| Tissue Location | Expressed in tumor endothelial cells but absent or barely detectable in normal endothelial cells. Expressed in metastatic lesions of the liver and during angiogenesis of corpus luteum formation and wound healing. Expressed in vascular endothelial cells of malignant tumors but not in normal blood vessels. Expressed in stromal fibroblasts. Strongly expressed in pericytes (PubMed:20484976) Expressed on stromal cells and cells with lymphoid morphology such a T- cells (PubMed:21466550). |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
TEM1 Antibody: Tumor endothelial marker (TEM) 1 was originally identified as a human embryonic fibroblast-specific antigen and was later determined to be endosialin, a single-pass transmembrane glycoprotein that has multiple extracellular domains, including three EGF-like domains, a sushi-like domain, and a C lectin-like domain. TEM proteins are significantly up-regulated during angiogenesis and neoangiogenesis that are crucial for the growth of solid tumors. While TEM1 is not required for angiogenesis during fetal development, postnatal growth or wound healing, it plays a role in tumor growth, invasion, and metastasis. Fibronectin and collagen types I and IV act as specific ligands of TEM1, leading to suggestions that these molecules may cause changes in the extracellular matrix, cell adhesion and migration during tumor invasion.
References
Rettig WJ, Garin-Chesa P, Healey JH, et al. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc. Natl. Acad. Sci. USA 1992; 89:10832-6.
Christian S, Ahorn H, Koehler A, et al. Molecular cloning and characterization of endosialin, a C-type lectin-like cell surface receptor of tumor endothelium. J. Biol. Chem. 2001; 276:7408-14.
Nanda A and St Croix B. Tumor endothelial markers: new targets for cancer therapy. Curr. Opin. Oncol. 2004; 16:44-9.
Nanda A, Karim B, Peng Z, et al. Tumor endothelial marker 1 (TEM1) functions in the growth and progression of abdominal tumors. Proc. Natl. Acad. Sci. USA 2006; 103:3351-6.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.