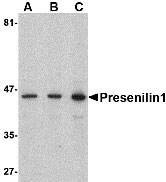
Presenilin1 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IHC-P, IF, E |
|---|---|
| Primary Accession | P49768 |
| Other Accession | NP_000012, 4506163 |
| Reactivity | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Calculated MW | 52668 Da |
| Application Notes | Presenilin1 antibody can be used for detection of presenilin1 by Western blot at 0.5 - 2 µg/mL. Antibody can also be used for immunohistochemistry starting at 2.5 µg/mL. For immunofluorescence start at 20 µg/mL. |
| Gene ID | 5663 |
|---|---|
| Other Names | Presenilin1 Antibody: AD3, FAD, PS1, PS-1, S182, AD3, PSNL1, Presenilin-1, Protein S182, presenilin 1 |
| Target/Specificity | PSEN1; |
| Reconstitution & Storage | Presenilin1 antibody can be stored at 4℃ for three months and -20℃, stable for up to one year. As with all antibodies care should be taken to avoid repeated freeze thaw cycles. Antibodies should not be exposed to prolonged high temperatures. |
| Precautions | Presenilin1 Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | PSEN1 |
|---|---|
| Synonyms | AD3, PS1, PSNL1 |
| Function | Catalytic subunit of the gamma-secretase complex, an endoprotease complex that catalyzes the intramembrane cleavage of integral membrane proteins such as Notch receptors and APP (amyloid- beta precursor protein) (PubMed:10206644, PubMed:10545183, PubMed:10593990, PubMed:10811883, PubMed:10899933, PubMed:12679784, PubMed:12740439, PubMed:15274632, PubMed:20460383, PubMed:25043039, PubMed:26280335, PubMed:28269784, PubMed:30598546, PubMed:30630874). Requires the presence of the other members of the gamma-secretase complex for protease activity (PubMed:15274632, PubMed:25043039, PubMed:26280335, PubMed:30598546, PubMed:30630874). Plays a role in Notch and Wnt signaling cascades and regulation of downstream processes via its role in processing key regulatory proteins, and by regulating cytosolic CTNNB1 levels (PubMed:10593990, PubMed:10811883, PubMed:10899933, PubMed:9738936). Stimulates cell-cell adhesion via its interaction with CDH1; this stabilizes the complexes between CDH1 (E- cadherin) and its interaction partners CTNNB1 (beta-catenin), CTNND1 and JUP (gamma-catenin) (PubMed:11953314). Under conditions of apoptosis or calcium influx, cleaves CDH1 (PubMed:11953314). This promotes the disassembly of the complexes between CDH1 and CTNND1, JUP and CTNNB1, increases the pool of cytoplasmic CTNNB1, and thereby negatively regulates Wnt signaling (PubMed:11953314, PubMed:9738936). Required for normal embryonic brain and skeleton development, and for normal angiogenesis (By similarity). Mediates the proteolytic cleavage of EphB2/CTF1 into EphB2/CTF2 (PubMed:17428795, PubMed:28269784). The holoprotein functions as a calcium-leak channel that allows the passive movement of calcium from endoplasmic reticulum to cytosol and is therefore involved in calcium homeostasis (PubMed:16959576, PubMed:25394380). Involved in the regulation of neurite outgrowth (PubMed:15004326, PubMed:20460383). Is a regulator of presynaptic facilitation, spike transmission and synaptic vesicles replenishment in a process that depends on gamma-secretase activity. It acts through the control of SYT7 presynaptic expression (By similarity). |
| Cellular Location | Endoplasmic reticulum. Endoplasmic reticulum membrane; Multi-pass membrane protein. Golgi apparatus membrane; Multi-pass membrane protein. Cytoplasmic granule. Cell membrane; Multi-pass membrane protein. Cell projection, growth cone. Early endosome. Early endosome membrane; Multi-pass membrane protein. Cell projection, neuron projection. Cell projection, axon {ECO:0000250|UniProtKB:Q4JIM4}. Synapse {ECO:0000250|UniProtKB:Q4JIM4}. Note=Translocates with bound NOTCH1 from the endoplasmic reticulum and/or Golgi to the cell surface (PubMed:10593990). Colocalizes with CDH1/2 at sites of cell-cell contact. Colocalizes with CTNNB1 in the endoplasmic reticulum and the proximity of the plasma membrane (PubMed:9738936). Also present in azurophil granules of neutrophils (PubMed:11987239). Colocalizes with UBQLN1 in the cell membrane and in cytoplasmic juxtanuclear structures called aggresomes (PubMed:21143716). |
| Tissue Location | Detected in azurophile granules in neutrophils and in platelet cytoplasmic granules (at protein level) (PubMed:11987239) Expressed in a wide range of tissues including various regions of the brain, liver, spleen and lymph nodes (PubMed:7596406, PubMed:8574969, PubMed:8641442). |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Presenilin1 Antibody: Presenilin1 was initially identified a marker of susceptibility to early-onset Alzheimer's disease. In addition to PEN2, nicastrin and APH-1, Presenilin1 forms the gamma-secretase protein complex, a membrane-bound aspartyl protease that can cleave certain proteins at peptide bonds buried within the hydrophobic environment of the lipid bilayer. This cleavage is responsible for a key step in signaling from several cell-surface receptors and is thought to be required for the generation of the neurotoxic amyloid peptides that are central to the pathogenesis of Alzheimer's disease. Like the tumor necrosis factor-alpha-converting enzyme (TACE) and the beta-site cleavage enzyme (BACE) protease families, gamma-secretase will cleave the amyloid precursor protein (APP), but within the intramembrane region of APP, resulting in either the non-toxic p3 (from the alpha and gamma cleavage site) or the toxic Abeta amyloid peptide (from the beta and gamma cleavage site). It is thought that accumulation of the Abeta peptide is the precursor to Alzheimer's disease. Multiple isoforms of presenilin1 are known to exist. This antibody has no cross-reactivity to presenilin2.
References
Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature1995; 375:754-60.
Weihofen A and Martoglio B. Intramembrane-cleaving proteases: controlled liberation of proteins and bioactive peptides. Trends Cell Biol.2003; 13:71-8.
Periz G and Fortini ME. Functional reconstitution of g-secretase through coordinated expression of presenilin, nicastrin, aph-1, and pen-2. J. Neurosci. Res.2004; 77:309-22.
Selkoe DJ. The cell biology of b-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol.1998; 8:447-53.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.