STUB1 Antibody (C-term)
Purified Rabbit Polyclonal Antibody (Pab)
- SPECIFICATION
- CITATIONS: 1
- PROTOCOLS
- BACKGROUND

Application
| WB, IHC-P, E |
|---|---|
| Primary Accession | Q9UNE7 |
| Other Accession | Q9WUD1, Q5ZHY5, Q969U2, A6HD62 |
| Reactivity | Human |
| Predicted | Chicken, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | Rabbit IgG |
| Calculated MW | 34856 Da |
| Antigen Region | 203-231 aa |
| Gene ID | 10273 |
|---|---|
| Other Names | E3 ubiquitin-protein ligase CHIP, 632-, Antigen NY-CO-7, CLL-associated antigen KW-8, Carboxy terminus of Hsp70-interacting protein, STIP1 homology and U box-containing protein 1 {ECO:0000312|HGNC:HGNC:11427}, STUB1 (HGNC:11427) |
| Target/Specificity | This STUB1 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 203-231 amino acids from the C-terminal region of human STUB1. |
| Dilution | WB~~1:1000 IHC-P~~1:50~100 |
| Format | Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is prepared by Saturated Ammonium Sulfate (SAS) precipitation followed by dialysis against PBS. |
| Storage | Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | STUB1 Antibody (C-term) is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | STUB1 {ECO:0000303|PubMed:23973223, ECO:0000312|HGNC:HGNC:11427} |
|---|---|
| Function | E3 ubiquitin-protein ligase which targets misfolded chaperone substrates towards proteasomal degradation (PubMed:10330192, PubMed:11146632, PubMed:11557750, PubMed:23990462, PubMed:26265139). Plays a role in the maintenance of mitochondrial morphology and promotes mitophagic removal of dysfunctional mitochondria; thereby acts as a protector against apoptosis in response to cellular stress (By similarity). Negatively regulates vascular smooth muscle contraction, via degradation of the transcriptional activator MYOCD and subsequent loss of transcription of genes involved in vascular smooth muscle contraction (By similarity). Promotes survival and proliferation of cardiac smooth muscle cells via ubiquitination and degradation of FOXO1, resulting in subsequent repression of FOXO1-mediated transcription of pro-apoptotic genes (PubMed:19483080). Ubiquitinates ICER-type isoforms of CREM and targets them for proteasomal degradation, thereby acts as a positive effector of MAPK/ERK-mediated inhibition of apoptosis in cardiomyocytes (PubMed:20724525). Inhibits lipopolysaccharide-induced apoptosis and hypertrophy in cardiomyocytes, via ubiquitination and subsequent proteasomal degradation of NFATC3 (PubMed:30980393). Collaborates with ATXN3 in the degradation of misfolded chaperone substrates: ATXN3 restricting the length of ubiquitin chain attached to STUB1/CHIP substrates and preventing further chain extension (PubMed:10330192, PubMed:11146632, PubMed:11557750, PubMed:23990462). Ubiquitinates NOS1 in concert with Hsp70 and Hsp40 (PubMed:15466472). Modulates the activity of several chaperone complexes, including Hsp70, Hsc70 and Hsp90 (PubMed:10330192, PubMed:11146632, PubMed:15466472). Ubiquitinates CHRNA3 targeting it for endoplasmic reticulum-associated degradation in cortical neurons, as part of the STUB1-VCP-UBXN2A complex (PubMed:26265139). Ubiquitinates and promotes ESR1 proteasomal degradation in response to age-related circulating estradiol (17-beta-estradiol/E2) decline, thereby promotes neuronal apoptosis in response to ischemic reperfusion injury (By similarity). Mediates transfer of non-canonical short ubiquitin chains to HSPA8 that have no effect on HSPA8 degradation (PubMed:11557750, PubMed:23990462). Mediates polyubiquitination of DNA polymerase beta (POLB) at 'Lys-41', 'Lys-61' and 'Lys-81', thereby playing a role in base-excision repair: catalyzes polyubiquitination by amplifying the HUWE1/ARF-BP1-dependent monoubiquitination and leading to POLB-degradation by the proteasome (PubMed:19713937). Mediates polyubiquitination of CYP3A4 (PubMed:19103148). Ubiquitinates EPHA2 and may regulate the receptor stability and activity through proteasomal degradation (PubMed:19567782). Acts as a co-chaperone for HSPA1A and HSPA1B chaperone proteins and promotes ubiquitin-mediated protein degradation (PubMed:27708256). Negatively regulates the suppressive function of regulatory T-cells (Treg) during inflammation by mediating the ubiquitination and degradation of FOXP3 in a HSPA1A/B-dependent manner (PubMed:23973223). Catalyzes monoubiquitination of SIRT6, preventing its degradation by the proteasome (PubMed:24043303). Likely mediates polyubiquitination and down-regulates plasma membrane expression of PD-L1/CD274, an immune inhibitory ligand critical for immune tolerance to self and antitumor immunity (PubMed:28813410). Negatively regulates TGF-beta signaling by modulating the basal level of SMAD3 via ubiquitin-mediated degradation (PubMed:24613385). Plays a role in the degradation of TP53 (PubMed:26634371). Mediates ubiquitination of RIPK3 leading to its subsequent proteasome-dependent degradation (PubMed:29883609). May regulate myosin assembly in striated muscles together with UBE4B and VCP/p97 by targeting myosin chaperone UNC45B for proteasomal degradation (PubMed:17369820). Ubiquitinates PPARG in macrophages playing a role in M2 macrophages polarization and angiogenesis (By similarity). |
| Cellular Location | Cytoplasm. Nucleus. Mitochondrion {ECO:0000250|UniProtKB:A6HD62}. Note=Translocates to the nucleus in response to inflammatory signals in regulatory T-cells (Treg) Localizes to mitochondria following oxygen and glucose deprivation- induced cellular stress (By similarity). {ECO:0000250|UniProtKB:A6HD62, ECO:0000269|PubMed:23973223} |
| Tissue Location | Expressed in differentiated myotubes (at protein level) (PubMed:17369820). Highly expressed in skeletal muscle, heart, pancreas, brain and placenta (PubMed:10330192, PubMed:11435423) Detected in kidney, liver and lung (PubMed:10330192, PubMed:11435423) |

Provided below are standard protocols that you may find useful for product applications.
Background
CHIP is an E3 ligase for nNOS whose action is facilitated by (and possibly requires) its interaction with nNOS-bound hsp70. Co-chaperone CHIP, possibly with another E3 ligase(s), modulates the ubiquitylation of mutant Cu/Zn-superoxide dismutase and renders them more susceptible for proteasomal degradation. CHIP functions as a negative regulator of AR transcriptional activity by promoting AR degradation. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. CHIP can interact with the Smad1/Smad4 proteins and block BMP signal transduction through the ubiquitin-mediated degradation of Smad proteins. CHIP E3 controls both the association of Hsp70/Hsp90 chaperones with ErbB2 and the down-regulation of ErbB2 induced by inhibitors of Hsp90. CHIP is associated with Parkin and enhances its ubiquitin ligase activity related to Parkinson's disease.
References
Peng,H.M., et al. J. Biol. Chem. 279 (51), 52970-52977 (2004)
Alberti,S., et al. Mol. Biol. Cell 15 (9), 4003-4010 (2004)
Beausoleil,S.A., et al. PNAS 101 (33), 12130-12135 (2004)
He,B., et al. J. Biol. Chem. 279 (29), 30643-30653 (2004)
Petrucelli,L., et al. Hum. Mol. Genet. 13 (7), 703-714 (2004)
Shimura,H., et al. J. Biol. Chem. 279 (6), 4869-4876 (2004)
Li,L., et al. Mol. Cell. Biol. 24 (2), 856-864 (2004)
Zhou,P., et al. J. Biol. Chem. 278 (16), 13829-13837 (2003)
Imai,Y., et al. Mol. Cell 10 (1), 55-67 (2002)
Ballinger,C.A., et al. Mol. Cell. Biol. 19 (6), 4535-4545 (1999)
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.





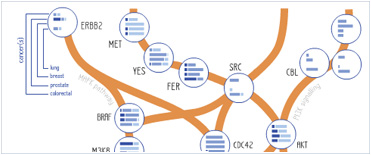







 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.




