Hsp90 Antibody
Purified Mouse Monoclonal Antibody (Mab)
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB |
|---|---|
| Primary Accession | P07900 |
| Reactivity | Human, Mouse |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG1 |
| Calculated MW | 90 KDa |
| Gene ID | 3320 |
|---|---|
| Other Names | D6S182;FLJ26984;FLJ31884;Heat shock 86 kDa;heat shock 90kDa protein 1 alpha;Heat shock protein 90kDa alpha cytosolic class A member 1;Heat shock protein 90kDa alpha cytosolic class B member 1; Heat shock protein HSP 90 alpha;Heat shock protein HSP 90 beta;Heat shock protein HSP 90-alpha;HS90A_HUMAN;HSP 84;HSP 86;Hsp 90;HSP84;HSP86;Hsp89;Hsp90;HSP90 Beta;HSP90A;HSP90AA1;HSP90AB1;HSP90B;HSP90N;HSPC1;HSPC2;HSPCA;HSPCAL1;HSPCAL4;HSPCB;HSPN;LAP2;Lipopolysaccharide associated protein2;LPS associated protein 2;NY REN 38 antigen;Renal carcinoma antigen NY-REN-38. |
| Dilution | WB~~1:2000 |
| Format | Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide, pH 7.3. |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Name | HSP90AA1 (HGNC:5253) |
|---|---|
| Synonyms | HSP90A, HSPC1, HSPCA |
| Function | Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function (PubMed:11274138, PubMed:12526792, PubMed:15577939, PubMed:15937123, PubMed:27353360, PubMed:29127155). Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself (PubMed:29127155). Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co- chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle (PubMed:26991466, PubMed:27295069). Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70 (PubMed:12526792). Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels (PubMed:25973397). In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues (PubMed:25973397). Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment (PubMed:25973397). Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression (PubMed:25973397). Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes (PubMed:11276205). Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation (PubMed:24613385). Mediates the association of TOMM70 with IRF3 or TBK1 in mitochondrial outer membrane which promotes host antiviral response (PubMed:20628368, PubMed:25609812). |
| Cellular Location | Nucleus {ECO:0000250|UniProtKB:P07901}. Cytoplasm {ECO:0000250|UniProtKB:P07901}. Melanosome. Cell membrane. Mitochondrion. Note=Identified by mass spectrometry in melanosome fractions from stage I to stage IV |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. Binds bacterial lipopolysaccharide (LPS) et mediates LPS-induced inflammatory response, including TNF secretion by monocytes.
References
Soeda E.,et al.Nucleic Acids Res. 17:7108-7108(1989).
Yamazaki M.,et al.Agric. Biol. Chem. 54:3163-3170(1990).
Hickey E.,et al.Mol. Cell. Biol. 9:2615-2626(1989).
Chen B.,et al.Genomics 86:627-637(2005).
Ota T.,et al.Nat. Genet. 36:40-45(2004).
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.




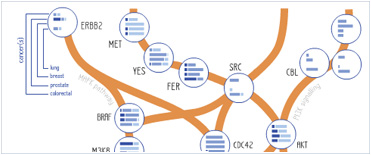







 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.


