HSPA5 Antibody (C-term)
Affinity Purified Rabbit Polyclonal Antibody (Pab)
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| FC, IHC-P, WB, E |
|---|---|
| Primary Accession | P11021 |
| Other Accession | Q91883, P06761, P34935, P20029, Q0VCX2, NP_005338, G3I8R9 |
| Reactivity | Human |
| Predicted | Bovine, Hamster, Mouse, Pig, Rat, Xenopus |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | Rabbit IgG |
| Calculated MW | 72333 Da |
| Antigen Region | 541-568 aa |
| Gene ID | 3309 |
|---|---|
| Other Names | 78 kDa glucose-regulated protein, GRP-78, Endoplasmic reticulum lumenal Ca(2+)-binding protein grp78, Heat shock 70 kDa protein 5, Immunoglobulin heavy chain-binding protein, BiP, HSPA5, GRP78 |
| Target/Specificity | This HSPA5 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 541-568 amino acids from the C-terminal region of human HSPA5. |
| Dilution | WB~~1:1000 IHC-P~~1:50~100 FC~~1:10~50 |
| Format | Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is purified through a protein A column, followed by peptide affinity purification. |
| Storage | Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | HSPA5 Antibody (C-term) is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | HSPA5 (HGNC:5238) |
|---|---|
| Function | Endoplasmic reticulum chaperone that plays a key role in protein folding and quality control in the endoplasmic reticulum lumen (PubMed:2294010, PubMed:23769672, PubMed:23990668, PubMed:28332555). Involved in the correct folding of proteins and degradation of misfolded proteins via its interaction with DNAJC10/ERdj5, probably to facilitate the release of DNAJC10/ERdj5 from its substrate (By similarity). Acts as a key repressor of the EIF2AK3/PERK and ERN1/IRE1- mediated unfolded protein response (UPR) (PubMed:1550958, PubMed:11907036, PubMed:19538957). In the unstressed endoplasmic reticulum, recruited by DNAJB9/ERdj4 to the luminal region of ERN1/IRE1, leading to disrupt the dimerization of ERN1/IRE1, thereby inactivating ERN1/IRE1 (By similarity). Also binds and inactivates EIF2AK3/PERK in unstressed cells (PubMed:11907036). Accumulation of misfolded protein in the endoplasmic reticulum causes release of HSPA5/BiP from ERN1/IRE1 and EIF2AK3/PERK, allowing their homodimerization and subsequent activation (PubMed:11907036). Plays an auxiliary role in post-translational transport of small presecretory proteins across endoplasmic reticulum (ER). May function as an allosteric modulator for SEC61 channel-forming translocon complex, likely cooperating with SEC62 to enable the productive insertion of these precursors into SEC61 channel. Appears to specifically regulate translocation of precursors having inhibitory residues in their mature region that weaken channel gating. May also play a role in apoptosis and cell proliferation (PubMed:26045166). |
| Cellular Location | Endoplasmic reticulum lumen. Melanosome. Cytoplasm {ECO:0000250|UniProtKB:P20029}. Cell surface Note=Identified by mass spectrometry in melanosome fractions from stage I to stage IV (PubMed:12643545). Localizes to the cell surface of epithelial cells in response to high levels of free iron (PubMed:20484814, PubMed:24355926, PubMed:27159390) |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
When Chinese hamster K12 cells are starved of glucose, the synthesis of several proteins, called glucose-regulated proteins (GRPs), is markedly increased. Hendershot et al. (1994) [PubMed 8020977] pointed out that one of these, GRP78 (HSPA5), also referred to as 'immunoglobulin heavy chain-binding protein' (BiP), is a member of the heat-shock protein-70 (HSP70) family and is involved in the folding and assembly of proteins in the endoplasmic reticulum (ER). Because so many ER proteins interact transiently with GRP78, it may play a key role in monitoring protein transport through the cell.
References
Zhao, C., et al. J. Med. Virol. 82(1):14-22(2010)
Zhuang, L., et al. Mod. Pathol. 23(1):45-53(2010)
Arnaudeau, S., et al. Proteomics 9(23):5316-5327(2009)
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.





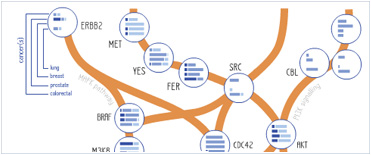







 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.




