R Csnk2a1 Antibody (N-term)
Affinity Purified Rabbit Polyclonal Antibody (Pab)
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, E |
|---|---|
| Primary Accession | P19139 |
| Other Accession | P08181, P18334, Q8NEV1, O54833, P19784, P21869, P20427, P28020, P33674, Q60737, P68400, P21868, P68399 |
| Reactivity | Human, Rat |
| Predicted | Bovine, Chicken, Mouse, Rabbit, Xenopus, C.Elegans, Drosophila |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | Rabbit IgG |
| Calculated MW | 45073 Da |
| Antigen Region | 28-50 aa |
| Gene ID | 116549 |
|---|---|
| Other Names | Casein kinase II subunit alpha, CK II alpha, Csnk2a1 |
| Target/Specificity | This Rat Csnk2a1 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 28-50 amino acids from the N-terminal region of Rat Csnk2a1. |
| Dilution | WB~~1:1000 |
| Format | Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is purified through a protein A column, followed by peptide affinity purification. |
| Storage | Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | R Csnk2a1 Antibody (N-term) is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | Csnk2a1 |
|---|---|
| Function | Catalytic subunit of a constitutively active serine/threonine-protein kinase complex that phosphorylates a large number of substrates containing acidic residues C-terminal to the phosphorylated serine or threonine (By similarity). Regulates numerous cellular processes, such as cell cycle progression, apoptosis and transcription, as well as viral infection (By similarity). May act as a regulatory node which integrates and coordinates numerous signals leading to an appropriate cellular response (By similarity). During mitosis, functions as a component of the p53/TP53-dependent spindle assembly checkpoint (SAC) that maintains cyclin-B-CDK1 activity and G2 arrest in response to spindle damage (By similarity). Also required for p53/TP53-mediated apoptosis, phosphorylating 'Ser-392' of p53/TP53 following UV irradiation (By similarity). Phosphorylates a number of DNA repair proteins in response to DNA damage, such as MDC1, MRE11, RAD9A, RAD51 and HTATSF1, promoting their recruitment to DNA damage sites (By similarity). Can also negatively regulate apoptosis (PubMed:12191471). Phosphorylates the caspases CASP9 and CASP2 and the apoptotic regulator NOL3 (PubMed:12191471). Phosphorylation protects CASP9 from cleavage and activation by CASP8, and inhibits the dimerization of CASP2 and activation of CASP8 (PubMed:12191471). Phosphorylates YY1, protecting YY1 from cleavage by CASP7 during apoptosis (By similarity). Regulates transcription by direct phosphorylation of RNA polymerases I, II, III and IV (By similarity). Also phosphorylates and regulates numerous transcription factors including NF-kappa-B, STAT1, CREB1, IRF1, IRF2, ATF1, ATF4, SRF, MAX, JUN, FOS, MYC and MYB (By similarity). Phosphorylates Hsp90 and its co- chaperones FKBP4 and CDC37, which is essential for chaperone function (By similarity). Mediates sequential phosphorylation of FNIP1, promoting its gradual interaction with Hsp90, leading to activate both kinase and non-kinase client proteins of Hsp90 (By similarity). Regulates Wnt signaling by phosphorylating CTNNB1 and the transcription factor LEF1 (By similarity). Acts as an ectokinase that phosphorylates several extracellular proteins (By similarity). Phosphorylates PML at 'Ser-565' and primes it for ubiquitin-mediated degradation (By similarity). Plays an important role in the circadian clock function by phosphorylating BMAL1 at 'Ser-90' which is pivotal for its interaction with CLOCK and which controls CLOCK nuclear entry (PubMed:19330005). Phosphorylates FMR1, promoting FMR1-dependent formation of a membraneless compartment (By similarity). May phosphorylate histone H2A on 'Ser-1' (By similarity). |
| Cellular Location | Nucleus {ECO:0000250|UniProtKB:P68400}. |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Catalytic subunit of a constitutively active serine/threonine-protein kinase complex that phosphorylates a large number of substrates containing acidic residues C-terminal to the phosphorylated serine or threonine. Regulates numerous cellular processes, such as cell cycle progression, apoptosis and transcription, as well as viral infection. May act as a regulatory node which integrates and coordinates numerous signals leading to an appropriate cellular response. During mitosis, functions as a component of the p53/TP53-dependent spindle assembly checkpoint (SAC) that maintains cyclin-B-CDK1 activity and G2 arrest in response to spindle damage. Also required for p53/TP53-mediated apoptosis, phosphorylating 'Ser-392' of p53/TP53 following UV irradiation. Can also negatively regulate apoptosis. Phosphorylates the caspases CASP9 and CASP2 and the apoptotic regulator NOL3. Phosphorylation protects CASP9 from cleavage and activation by CASP8, and inhibits the dimerization of CASP2 and activation of CASP8. Regulates transcription by direct phosphorylation of RNA polymerases I, II, III and IV. Also phosphorylates and regulates numerous transcription factors including NF-kappa-B, STAT1, CREB1, IRF1, IRF2, ATF1, SRF, MAX, JUN, FOS, MYC and MYB. Phosphorylates Hsp90 and its co-chaperones FKBP4 and CDC37, which is essential for chaperone function. Regulates Wnt signaling by phosphorylating CTNNB1 and the transcription factor LEF1. Acts as an ectokinase that phosphorylates several extracellular proteins.
References
Ahmed K., et al. Cell. Mol. Biol. Res. 39:451-462(1993).
Meisner H., et al. Biochemistry 28:4072-4076(1989).
Li P.F., et al. Mol. Cell 10:247-258(2002).
Zhou W., et al. Chin. Sci. Bull. 54:220-226(2009).
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.




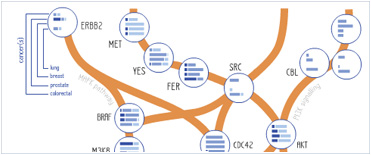







 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.


