FAP Antibody
Purified Rabbit Polyclonal Antibody (Pab)
- SPECIFICATION
- CITATIONS: 1
- PROTOCOLS
- BACKGROUND

Application
| WB, E |
|---|---|
| Primary Accession | Q12884 |
| Other Accession | NP_004451.2 |
| Reactivity | Human |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | Rabbit IgG |
| Calculated MW | 87713 Da |
| Gene ID | 2191 |
|---|---|
| Other Names | Prolyl endopeptidase FAP, 170 kDa melanoma membrane-bound gelatinase, Dipeptidyl peptidase FAP, Fibroblast activation protein alpha, FAPalpha, Serine integral membrane protease, SIMP, Surface-expressed protease, Seprase, Antiplasmin-cleaving enzyme FAP, soluble form, APCE, 3421-, FAP (HGNC:3590) |
| Target/Specificity | This FAP antibody is generated from rabbits immunized with the protein of human FAP. |
| Dilution | WB~~1:1000 |
| Format | Purified monoclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is purified through a protein A column, followed by dialysis against PBS. |
| Storage | Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | FAP Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | FAP (HGNC:3590) |
|---|---|
| Function | Cell surface glycoprotein serine protease that participates in extracellular matrix degradation and involved in many cellular processes including tissue remodeling, fibrosis, wound healing, inflammation and tumor growth. Both plasma membrane and soluble forms exhibit post-proline cleaving endopeptidase activity, with a marked preference for Ala/Ser-Gly-Pro-Ser/Asn/Ala consensus sequences, on substrate such as alpha-2-antiplasmin SERPINF2 and SPRY2 (PubMed:14751930, PubMed:16223769, PubMed:16410248, PubMed:16480718, PubMed:17381073, PubMed:18095711, PubMed:21288888, PubMed:24371721). Degrade also gelatin, heat-denatured type I collagen, but not native collagen type I and IV, vitronectin, tenascin, laminin, fibronectin, fibrin or casein (PubMed:10347120, PubMed:10455171, PubMed:12376466, PubMed:16223769, PubMed:16651416, PubMed:18095711, PubMed:2172980, PubMed:7923219, PubMed:9065413). Also has dipeptidyl peptidase activity, exhibiting the ability to hydrolyze the prolyl bond two residues from the N-terminus of synthetic dipeptide substrates provided that the penultimate residue is proline, with a preference for Ala-Pro, Ile-Pro, Gly-Pro, Arg-Pro and Pro-Pro (PubMed:10347120, PubMed:10593948, PubMed:16175601, PubMed:16223769, PubMed:16410248, PubMed:16651416, PubMed:17381073, PubMed:21314817, PubMed:24371721, PubMed:24717288). Natural neuropeptide hormones for dipeptidyl peptidase are the neuropeptide Y (NPY), peptide YY (PYY), substance P (TAC1) and brain natriuretic peptide 32 (NPPB) (PubMed:21314817). The plasma membrane form, in association with either DPP4, PLAUR or integrins, is involved in the pericellular proteolysis of the extracellular matrix (ECM), and hence promotes cell adhesion, migration and invasion through the ECM. Plays a role in tissue remodeling during development and wound healing. Participates in the cell invasiveness towards the ECM in malignant melanoma cancers. Enhances tumor growth progression by increasing angiogenesis, collagen fiber degradation and apoptosis and by reducing antitumor response of the immune system. Promotes glioma cell invasion through the brain parenchyma by degrading the proteoglycan brevican. Acts as a tumor suppressor in melanocytic cells through regulation of cell proliferation and survival in a serine protease activity-independent manner. |
| Cellular Location | [Prolyl endopeptidase FAP]: Cell surface. Cell membrane; Single-pass type II membrane protein. Cell projection, lamellipodium membrane; Single-pass type II membrane protein. Cell projection, invadopodium membrane; Single-pass type II membrane protein. Cell projection, ruffle membrane; Single-pass type II membrane protein. Membrane; Single- pass type II membrane protein. Note=Localized on cell surface with lamellipodia and invadopodia membranes and on shed vesicles. Colocalized with DPP4 at invadopodia and lamellipodia membranes of migratory activated endothelial cells in collagenous matrix. Colocalized with DPP4 on endothelial cells of capillary-like microvessels but not large vessels within invasive breast ductal carcinoma. Anchored and enriched preferentially by integrin alpha- 3/beta-1 at invadopodia, plasma membrane protrusions that correspond to sites of cell invasion, in a collagen-dependent manner. Localized at plasma and ruffle membranes in a collagen-independent manner Colocalized with PLAUR preferentially at the cell surface of invadopodia membranes in a cytoskeleton-, integrin- and vitronectin- dependent manner. Concentrated at invadopodia membranes, specialized protrusions of the ventral plasma membrane in a fibrobectin-dependent manner. Colocalizes with extracellular components (ECM), such as collagen fibers and fibronectin. [Isoform 2]: Cytoplasm |
| Tissue Location | Expressed in adipose tissue. Expressed in the dermal fibroblasts in the fetal skin. Expressed in the granulation tissue of healing wounds and on reactive stromal fibroblast in epithelial cancers. Expressed in activated fibroblast-like synoviocytes from inflamed synovial tissues. Expressed in activated hepatic stellate cells (HSC) and myofibroblasts from cirrhotic liver, but not detected in normal liver. Expressed in glioma cells (at protein level) Expressed in glioblastomas and glioma cells. Isoform 1 and isoform 2 are expressed in melanoma, carcinoma and fibroblast cell lines |

Provided below are standard protocols that you may find useful for product applications.
Background
The protein encoded by this gene is a homodimeric integral membrane gelatinase belonging to the serine protease family. It is selectively expressed in reactive stromal fibroblasts of epithelial cancers, granulation tissue of healing wounds, and malignant cells of bone and soft tissue sarcomas. This protein is thought to be involved in the control of fibroblast growth or epithelial-mesenchymal interactions during development, tissue repair, and epithelial carcinogenesis.
References
Rose, J.E., et al. Mol. Med. 16 (7-8), 247-253 (2010) : Ospelt, C., et al. Arthritis Rheum. 62(5):1224-1235(2010) Stremenova, J., et al. Int. J. Oncol. 36(2):351-358(2010) Chen, H., et al. Exp. Mol. Pathol. 87(3):189-194(2009) Dohi, O., et al. Histopathology 55(4):432-440(2009)
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.






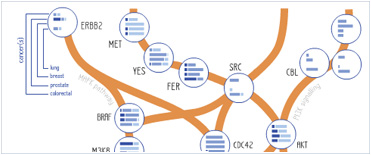







 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.

