PTGS2 Antibody (Center P378)
Affinity Purified Rabbit Polyclonal Antibody (Pab)
- SPECIFICATION
- CITATIONS: 1
- PROTOCOLS
- BACKGROUND

Application
| WB, IHC-P, IF, E |
|---|---|
| Primary Accession | P35354 |
| Other Accession | NP_000954.1 |
| Reactivity | Human |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | Rabbit IgG |
| Calculated MW | 68996 Da |
| Antigen Region | 363-391 aa |
| Gene ID | 5743 |
|---|---|
| Other Names | Prostaglandin G/H synthase 2, Cyclooxygenase-2, COX-2, PHS II, Prostaglandin H2 synthase 2, PGH synthase 2, PGHS-2, Prostaglandin-endoperoxide synthase 2, PTGS2, COX2 |
| Target/Specificity | This PTGS2 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 363-391 amino acids from the Central region of human PTGS2. |
| Dilution | IF~~1:10~50 WB~~1:2000 IHC-P~~1:10~50 |
| Format | Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is purified through a protein A column, followed by peptide affinity purification. |
| Storage | Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | PTGS2 Antibody (Center P378) is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | PTGS2 (HGNC:9605) |
|---|---|
| Function | Dual cyclooxygenase and peroxidase in the biosynthesis pathway of prostanoids, a class of C20 oxylipins mainly derived from arachidonate ((5Z,8Z,11Z,14Z)-eicosatetraenoate, AA, C20:4(n-6)), with a particular role in the inflammatory response (PubMed:11939906, PubMed:16373578, PubMed:19540099, PubMed:22942274, PubMed:26859324, PubMed:27226593, PubMed:7592599, PubMed:7947975, PubMed:9261177). The cyclooxygenase activity oxygenates AA to the hydroperoxy endoperoxide prostaglandin G2 (PGG2), and the peroxidase activity reduces PGG2 to the hydroxy endoperoxide prostaglandin H2 (PGH2), the precursor of all 2-series prostaglandins and thromboxanes (PubMed:16373578, PubMed:22942274, PubMed:26859324, PubMed:27226593, PubMed:7592599, PubMed:7947975, PubMed:9261177). This complex transformation is initiated by abstraction of hydrogen at carbon 13 (with S- stereochemistry), followed by insertion of molecular O2 to form the endoperoxide bridge between carbon 9 and 11 that defines prostaglandins. The insertion of a second molecule of O2 (bis-oxygenase activity) yields a hydroperoxy group in PGG2 that is then reduced to PGH2 by two electrons (PubMed:16373578, PubMed:22942274, PubMed:26859324, PubMed:27226593, PubMed:7592599, PubMed:7947975, PubMed:9261177). Similarly catalyzes successive cyclooxygenation and peroxidation of dihomo-gamma-linoleate (DGLA, C20:3(n-6)) and eicosapentaenoate (EPA, C20:5(n-3)) to corresponding PGH1 and PGH3, the precursors of 1- and 3-series prostaglandins (PubMed:11939906, PubMed:19540099). In an alternative pathway of prostanoid biosynthesis, converts 2-arachidonoyl lysophopholipids to prostanoid lysophopholipids, which are then hydrolyzed by intracellular phospholipases to release free prostanoids (PubMed:27642067). Metabolizes 2-arachidonoyl glycerol yielding the glyceryl ester of PGH2, a process that can contribute to pain response (PubMed:22942274). Generates lipid mediators from n-3 and n-6 polyunsaturated fatty acids (PUFAs) via a lipoxygenase-type mechanism. Oxygenates PUFAs to hydroperoxy compounds and then reduces them to corresponding alcohols (PubMed:11034610, PubMed:11192938, PubMed:9048568, PubMed:9261177). Plays a role in the generation of resolution phase interaction products (resolvins) during both sterile and infectious inflammation (PubMed:12391014). Metabolizes docosahexaenoate (DHA, C22:6(n-3)) to 17R-HDHA, a precursor of the D-series resolvins (RvDs) (PubMed:12391014). As a component of the biosynthetic pathway of E- series resolvins (RvEs), converts eicosapentaenoate (EPA, C20:5(n-3)) primarily to 18S-HEPE that is further metabolized by ALOX5 and LTA4H to generate 18S-RvE1 and 18S-RvE2 (PubMed:21206090). In vascular endothelial cells, converts docosapentaenoate (DPA, C22:5(n-3)) to 13R- HDPA, a precursor for 13-series resolvins (RvTs) shown to activate macrophage phagocytosis during bacterial infection (PubMed:26236990). In activated leukocytes, contributes to oxygenation of hydroxyeicosatetraenoates (HETE) to diHETES (5,15-diHETE and 5,11- diHETE) (PubMed:22068350, PubMed:26282205). Can also use linoleate (LA, (9Z,12Z)-octadecadienoate, C18:2(n-6)) as substrate and produce hydroxyoctadecadienoates (HODEs) in a regio- and stereospecific manner, being (9R)-HODE ((9R)-hydroxy-(10E,12Z)-octadecadienoate) and (13S)- HODE ((13S)-hydroxy-(9Z,11E)-octadecadienoate) its major products (By similarity). During neuroinflammation, plays a role in neuronal secretion of specialized preresolving mediators (SPMs) 15R-lipoxin A4 that regulates phagocytic microglia (By similarity). |
| Cellular Location | Microsome membrane; Peripheral membrane protein. Endoplasmic reticulum membrane; Peripheral membrane protein. Nucleus inner membrane; Peripheral membrane protein. Nucleus outer membrane; Peripheral membrane protein. Note=Detected on the lumenal side of the endoplasmic reticulum and nuclear envelope |

Provided below are standard protocols that you may find useful for product applications.
Background
Prostaglandin-endoperoxide synthase (PTGS), also known as cyclooxygenase, is the key enzyme in prostaglandin biosynthesis, and acts both as a dioxygenase and as a peroxidase. There are two isozymes of PTGS: a constitutive PTGS1 and an inducible PTGS2, which differ in their regulation of expression and tissue distribution. This gene encodes the inducible isozyme. It is regulated by specific stimulatory events, suggesting that it is responsible for the prostanoid biosynthesis involved in inflammation and mitogenesis.
References
Duggan, K.C., et al. J. Biol. Chem. 285(45):34950-34959(2010)
Feher, A., et al. Am J Geriatr Psychiatry 18(11):983-987(2010)
Wang, C.H., et al. Anticancer Res. 30(9):3649-3653(2010)
Han, E.H., et al. J. Toxicol. Environ. Health Part A 73 (21-22), 1451-1464 (2010) :
Cao, H., et al. Tohoku J. Exp. Med. 222(1):15-21(2010)
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.






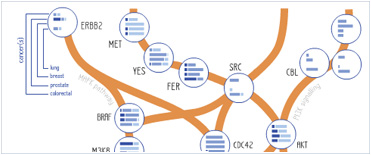







 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.




