TGM2 antibody ( Ascites)
Mouse Monoclonal Antibody (Mab)
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, E |
|---|---|
| Primary Accession | P21980 |
| Reactivity | Human |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG1 |
| Clone/Animal Names | 207CT39.5.5 |
| Calculated MW | 77329 Da |
| Gene ID | 7052 |
|---|---|
| Other Names | Protein-glutamine gamma-glutamyltransferase 2, Tissue transglutaminase, Transglutaminase C, TG(C), TGC, TGase C, Transglutaminase H, TGase H, Transglutaminase-2, TGase-2, TGM2 |
| Target/Specificity | This TGM2 monoclonal antibody is generated from mouse immunized with TGM2 recombinant protein. |
| Dilution | WB~~1:1000 |
| Format | Mouse monoclonal antibody supplied in crude ascites with 0.09% (W/V) sodium azide. |
| Storage | Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | TGM2 antibody ( Ascites) is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | TGM2 {ECO:0000303|PubMed:17939176, ECO:0000312|HGNC:HGNC:11778} |
|---|---|
| Function | Calcium-dependent acyltransferase that catalyzes the formation of covalent bonds between peptide-bound glutamine and various primary amines, such as gamma-amino group of peptide-bound lysine, or mono- and polyamines, thereby producing cross-linked or aminated proteins, respectively (PubMed:23941696, PubMed:31991788, PubMed:9252372). Involved in many biological processes, such as bone development, angiogenesis, wound healing, cellular differentiation, chromatin modification and apoptosis (PubMed:1683874, PubMed:27270573, PubMed:28198360, PubMed:7935379, PubMed:9252372). Acts as a protein- glutamine gamma-glutamyltransferase by mediating the cross-linking of proteins, such as ACO2, HSPB6, FN1, HMGB1, RAP1GDS1, SLC25A4/ANT1, SPP1 and WDR54 (PubMed:23941696, PubMed:24349085, PubMed:29618516, PubMed:30458214). Under physiological conditions, the protein cross- linking activity is inhibited by GTP; inhibition is relieved by Ca(2+) in response to various stresses (PubMed:18092889, PubMed:7592956, PubMed:7649299). When secreted, catalyzes cross-linking of proteins of the extracellular matrix, such as FN1 and SPP1 resulting in the formation of scaffolds (PubMed:12506096). Plays a key role during apoptosis, both by (1) promoting the cross-linking of cytoskeletal proteins resulting in condensation of the cytoplasm, and by (2) mediating cross-linking proteins of the extracellular matrix, resulting in the irreversible formation of scaffolds that stabilize the integrity of the dying cells before their clearance by phagocytosis, thereby preventing the leakage of harmful intracellular components (PubMed:7935379, PubMed:9252372). In addition to protein cross-linking, can use different monoamine substrates to catalyze a vast array of protein post-translational modifications: mediates aminylation of serotonin, dopamine, noradrenaline or histamine into glutamine residues of target proteins to generate protein serotonylation, dopaminylation, noradrenalinylation or histaminylation, respectively (PubMed:23797785, PubMed:30867594). Mediates protein serotonylation of small GTPases during activation and aggregation of platelets, leading to constitutive activation of these GTPases (By similarity). Plays a key role in chromatin organization by mediating serotonylation and dopaminylation of histone H3 (PubMed:30867594, PubMed:32273471). Catalyzes serotonylation of 'Gln-5' of histone H3 (H3Q5ser) during serotonergic neuron differentiation, thereby facilitating transcription (PubMed:30867594). Acts as a mediator of neurotransmission-independent role of nuclear dopamine in ventral tegmental area (VTA) neurons: catalyzes dopaminylation of 'Gln-5' of histone H3 (H3Q5dop), thereby regulating relapse-related transcriptional plasticity in the reward system (PubMed:32273471). Regulates vein remodeling by mediating serotonylation and subsequent inactivation of ATP2A2/SERCA2 (By similarity). Also acts as a protein deamidase by mediating the side chain deamidation of specific glutamine residues of proteins to glutamate (PubMed:20547769, PubMed:9623982). Catalyzes specific deamidation of protein gliadin, a component of wheat gluten in the diet (PubMed:9623982). May also act as an isopeptidase cleaving the previously formed cross-links (PubMed:26250429, PubMed:27131890). Also able to participate in signaling pathways independently of its acyltransferase activity: acts as a signal transducer in alpha-1 adrenergic receptor-mediated stimulation of phospholipase C-delta (PLCD) activity and is required for coupling alpha-1 adrenergic agonists to the stimulation of phosphoinositide lipid metabolism (PubMed:8943303). |
| Cellular Location | Cytoplasm, cytosol. Nucleus. Chromosome. Secreted, extracellular space, extracellular matrix. Cell membrane {ECO:0000250|UniProtKB:Q9WVJ6}. Mitochondrion. Note=Mainly localizes to the cytosol (PubMed:9575137). Present at much lower level in the nucleus and chromatin (PubMed:9575137). Also secreted via a non-classical secretion pathway to the extracellular matrix (PubMed:27270573) |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Transglutaminases are enzymes that catalyze the crosslinking of proteins by epsilon-gamma glutamyl lysine isopeptide bonds. While the primary structure of transglutaminases is not conserved, they all have the same amino acid sequence at their active sites and their activity is calcium-dependent. The protein encoded by this gene acts as a monomer, is induced by retinoic acid, and appears to be involved in apoptosis. Finally, the encoded protein is the autoantigen implicated in celiac disease. Two transcript variants encoding different isoforms have been found for this gene.
References
Munezane, T., et al. J. Vasc. Surg. 52(4):967-974(2010)
Kim, D.S., et al. Biochem. Biophys. Res. Commun. 399(2):300-306(2010)
Stamnaes, J., et al. J. Biol. Chem. 285(33):25402-25409(2010)
Santhanam, L., et al. Circ. Res. 107(1):117-125(2010)
Kumar, A., et al. PLoS ONE 5 (10), E13390 (2010) :
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.




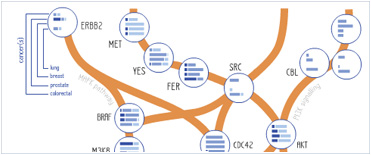







 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.


