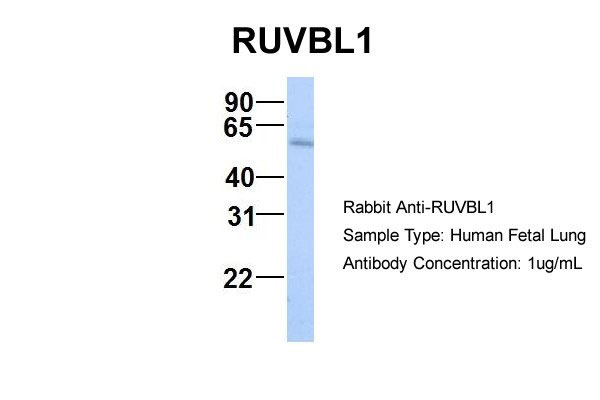
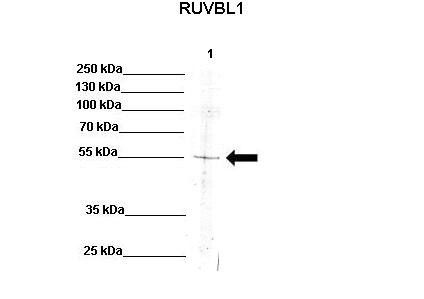
RUVBL1 antibody - N-terminal region
Rabbit Polyclonal Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB |
|---|---|
| Primary Accession | Q9Y265 |
| Other Accession | Q9Y265, NP_003698, NM_003707 |
| Reactivity | Human, Mouse, Rat, Rabbit, Zebrafish, Pig, Goat, Dog, Guinea Pig, Horse, Bovine |
| Predicted | Human, Mouse, Rabbit, Zebrafish, Chicken, Dog, Horse, Bovine |
| Host | Rabbit |
| Clonality | Polyclonal |
| Calculated MW | 50 kDa |
| Gene ID | 8607 |
|---|---|
| Alias Symbol | ECP54, NMP238, Pontin52, RVB1, TIH1, TIP49, TIP49A, INO80H, PONTIN |
| Other Names | RuvB-like 1, 49 kDa TATA box-binding protein-interacting protein, 49 kDa TBP-interacting protein, 54 kDa erythrocyte cytosolic protein, ECP-54, INO80 complex subunit H, Nuclear matrix protein 238, NMP 238, Pontin 52, TIP49a, TIP60-associated protein 54-alpha, TAP54-alpha, RUVBL1, INO80H, NMP238, TIP49, TIP49A |
| Target/Specificity | RUVBL1 possesses single-stranded DNA-stimulated ATPase and ATP-dependent DNA helicase (3' to 5') activity. It is the component of the NuA4 histone acetyltransferase complex which is involved in transcriptional activation of select genes principally by acetylation of nucleosomal histone H4 and H2A. The NuA4 complex ATPase and helicase activities seem to be, at least in part, contributed by the association of RUVBL1 and RUVBL2 with EP400. NuA4 may also play a direct role in DNA repair when recruited to sites of DNA damage. RUVBL1 plays an essential role in oncogenic transformation by MYC and also modulates transcriptional activation by the LEF1/TCF1-CTNNB1 complex. |
| Format | Liquid. Purified antibody supplied in 1x PBS buffer with 0.09% (w/v) sodium azide and 2% sucrose. |
| Reconstitution & Storage | Add 50 ul of distilled water. Final anti-RUVBL1 antibody concentration is 1 mg/ml in PBS buffer with 2% sucrose. For longer periods of storage, store at -20°C. Avoid repeat freeze-thaw cycles. |
| Precautions | RUVBL1 antibody - N-terminal region is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | RUVBL1 (HGNC:10474) |
|---|---|
| Function | Possesses single-stranded DNA-stimulated ATPase and ATP- dependent DNA helicase (3' to 5') activity; hexamerization is thought to be critical for ATP hydrolysis and adjacent subunits in the ring- like structure contribute to the ATPase activity (PubMed:17157868, PubMed:33205750). Component of the NuA4 histone acetyltransferase complex which is involved in transcriptional activation of select genes principally by acetylation of nucleosomal histones H4 and H2A (PubMed:14966270). This modification may both alter nucleosome-DNA interactions and promote interaction of the modified histones with other proteins which positively regulate transcription (PubMed:14966270). This complex may be required for the activation of transcriptional programs associated with oncogene and proto-oncogene mediated growth induction, tumor suppressor mediated growth arrest and replicative senescence, apoptosis, and DNA repair (PubMed:14966270). The NuA4 complex ATPase and helicase activities seem to be, at least in part, contributed by the association of RUVBL1 and RUVBL2 with EP400. NuA4 may also play a direct role in DNA repair when recruited to sites of DNA damage (PubMed:14966270). Component of a SWR1-like complex that specifically mediates the removal of histone H2A.Z/H2AZ1 from the nucleosome (PubMed:24463511). Proposed core component of the chromatin remodeling INO80 complex which exhibits DNA- and nucleosome-activated ATPase activity and catalyzes ATP-dependent nucleosome sliding (PubMed:16230350, PubMed:21303910). Plays an essential role in oncogenic transformation by MYC and also modulates transcriptional activation by the LEF1/TCF1-CTNNB1 complex (PubMed:10882073, PubMed:16014379). Essential for cell proliferation (PubMed:14506706). May be able to bind plasminogen at cell surface and enhance plasminogen activation (PubMed:11027681). |
| Cellular Location | Nucleus matrix. Nucleus, nucleoplasm. Cytoplasm. Membrane Cytoplasm, cytoskeleton, microtubule organizing center, centrosome Dynein axonemal particle {ECO:0000250|UniProtKB:Q9DE26}. Note=Mainly localized in the nucleus, associated with nuclear matrix or in the nuclear cytosol, although it is also present in the cytoplasm and associated with the cell membranes. In prophase and prometaphase it is located at the centrosome and the branching microtubule spindles. After mitotic nuclear membrane disintigration it accumulates at the centrosome and sites of tubulin polymerization. As cells pass through metaphase and into telophase it is located close to the centrosome at the early phase of tubulin polymerization. In anaphase it accumulates at the zone of tubule interdigitation. In telophase it is found at polar tubule overlap, and it reappears at the site of chromosomal decondensation in the daughter cells |
| Tissue Location | Ubiquitously expressed with high expression in heart, skeletal muscle and testis |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
This is a rabbit polyclonal antibody against RUVBL1. It was validated on Western Blot using a cell lysate as a positive control. Abgent strives to provide antibodies covering each member of a whole protein family of your interest. We also use our best efforts to provide you antibodies recognize various epitopes of a target protein. For availability of antibody needed for your experiment, please inquire (sales@abgent.com).
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.