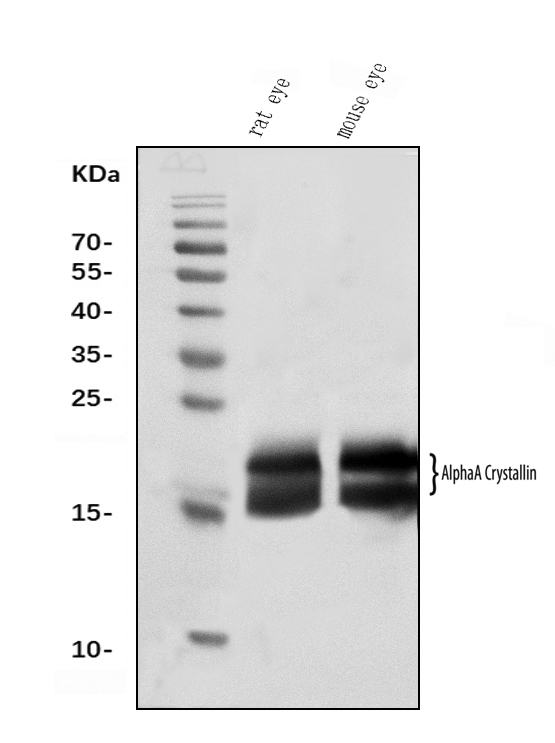
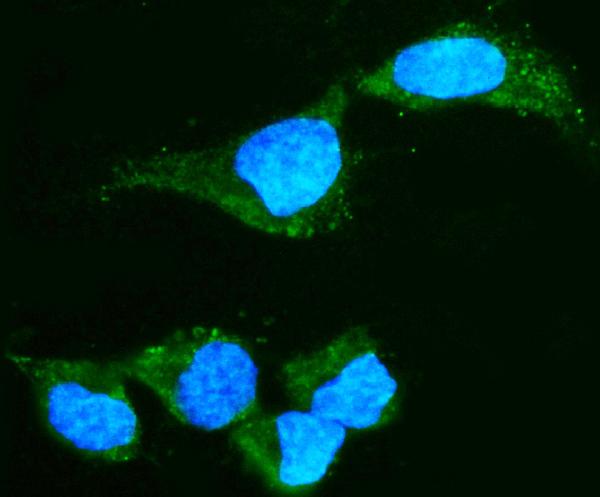
Anti-Alpha A Crystallin Antibody Picoband™ (monoclonal, 10B9)
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IF, ICC |
|---|---|
| Primary Accession | P02489 |
| Host | Mouse |
| Isotype | Mouse IgG1 |
| Reactivity | Rat, Human, Mouse |
| Clonality | Monoclonal |
| Format | Lyophilized |
| Description | Anti-Alpha A Crystallin Antibody Picoband™ (monoclonal, 10B9) . Tested in IF, ICC, WB applications. This antibody reacts with Human, Mouse, Rat. |
| Reconstitution | Adding 0.2 ml of distilled water will yield a concentration of 500 µg/ml. |
| Gene ID | 102724652;1409 |
|---|---|
| Other Names | Alpha-crystallin A chain, Heat shock protein beta-4, HspB4, Heat shock protein family B member 4, Alpha-crystallin A(1-172), Alpha-crystallin A(1-168), Alpha-crystallin A(1-162), CRYAA, CRYA1, HSPB4 |
| Calculated MW | 20-23 kDa |
| Application Details | Western blot, 0.25-0.5 µg/ml, Mouse, Rat Immunocytochemistry/Immunofluorescence, 5 µg/ml, Human |
| Contents | Each vial contains 4 mg Trehalose, 0.9 mg NaCl and 0.2 mg Na2HPO4. |
| Clone Names | Clone: 10B9 |
| Immunogen | E. coli-derived human Alpha A Crystallin recombinant protein (Position: M1-S173). Human Alpha A Crystallin shares 94.8% amino acid (aa) sequence identity with both mouse and rat Alpha A Crystallin. |
| Purification | Immunogen affinity purified. |
| Storage | At -20°C for one year from date of receipt. After reconstitution, at 4°C for one month. It can also be aliquotted and stored frozen at -20°C for six months. Avoid repeated freezing and thawing. |
| Name | CRYAA |
|---|---|
| Synonyms | CRYA1, HSPB4 |
| Function | Contributes to the transparency and refractive index of the lens (PubMed:18302245). In its oxidized form (absence of intramolecular disulfide bond), acts as a chaperone, preventing aggregation of various proteins under a wide range of stress conditions (PubMed:18199971, PubMed:19595763, PubMed:22120592, PubMed:31792453). Required for the correct formation of lens intermediate filaments as part of a complex composed of BFSP1, BFSP2 and CRYAA (PubMed:28935373). |
| Cellular Location | Cytoplasm. Nucleus. Note=Translocates to the nucleus during heat shock and resides in sub-nuclear structures known as SC35 speckles or nuclear splicing speckles |
| Tissue Location | Expressed in the eye lens (at protein level). |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Alpha-crystallin A chain is a protein that in humans is encoded by the CRYAA gene. Mammalian lens crystallins are divided into alpha, beta, and gamma families. Alpha crystallins are composed of two gene products: alpha-A and alpha-B, for acidic and basic, respectively. Alpha crystallins can be induced by heat shock and are members of the small heat shock protein (HSP20) family. They act as molecular chaperones although they do not renature proteins and release them in the fashion of a true chaperone; instead they hold them in large soluble aggregates. Two additional functions of alpha crystallins are an autokinase activity and participation in the intracellular architecture. The encoded protein has been identified as a moonlighting protein based on its ability to perform mechanistically distinct functions. Alpha-A and alpha-B gene products are differentially expressed; alpha-A is preferentially restricted to the lens and alpha-B is expressed widely in many tissues and organs. Defects in this gene cause autosomal dominant congenital cataract (ADCC).
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.