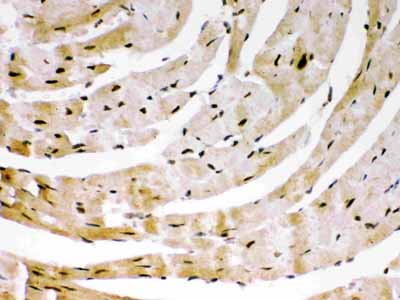
Anti-SIRT6 Picoband Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IHC-P |
|---|---|
| Primary Accession | Q8N6T7 |
| Host | Rabbit |
| Reactivity | Human, Mouse |
| Clonality | Polyclonal |
| Format | Lyophilized |
| Description | Rabbit IgG polyclonal antibody for NAD-dependent protein deacetylase sirtuin-6(SIRT6) detection. Tested with WB, IHC-P in Human;Mouse. |
| Reconstitution | Add 0.2ml of distilled water will yield a concentration of 500ug/ml. |
| Gene ID | 51548 |
|---|---|
| Other Names | NAD-dependent protein deacetylase sirtuin-6, 3.5.1.-, Regulatory protein SIR2 homolog 6, SIR2-like protein 6, SIRT6, SIR2L6 |
| Calculated MW | 39119 MW KDa |
| Application Details | Immunohistochemistry(Paraffin-embedded Section), 0.5-1 µg/ml, Human, Mouse, By Heat Western blot, 0.1-0.5 µg/ml, Human |
| Subcellular Localization | Nucleus, nucleoplasm . Predominantly nuclear. Associated with telomeric heterochromatin regions. |
| Protein Name | NAD-dependent protein deacetylase sirtuin-6 |
| Contents | Each vial contains 5mg BSA, 0.9mg NaCl, 0.2mg Na2HPO4, 0.05mg NaN3. |
| Immunogen | E.coli-derived human SIRT6 recombinant protein (Position: D14-E180). Human SIRT6 shares 95% amino acid (aa) sequence identity with mouse SIRT6. |
| Purification | Immunogen affinity purified. |
| Cross Reactivity | No cross reactivity with other proteins. |
| Storage | At -20˚C for one year. After r˚Constitution, at 4˚C for one month. It˚Can also be aliquotted and stored frozen at -20˚C for a longer time.Avoid repeated freezing and thawing. |
| Sequence Similarities | Belongs to the sirtuin family. Class IV subfamily. |
| Name | SIRT6 {ECO:0000303|PubMed:10873683, ECO:0000312|HGNC:HGNC:14934} |
|---|---|
| Function | NAD-dependent protein deacetylase, deacylase and mono-ADP- ribosyltransferase that plays an essential role in DNA damage repair, telomere maintenance, metabolic homeostasis, inflammation, tumorigenesis and aging (PubMed:18337721, PubMed:19135889, PubMed:19625767, PubMed:21362626, PubMed:21680843, PubMed:23217706, PubMed:23552949, PubMed:23653361, PubMed:24052263, PubMed:27180906, PubMed:27322069, PubMed:29555651, PubMed:30374165). Displays protein- lysine deacetylase or defatty-acylase (demyristoylase and depalmitoylase) activity, depending on the context (PubMed:23552949, PubMed:24052263, PubMed:27322069). Acts as a key histone deacetylase by catalyzing deacetylation of histone H3 at 'Lys-9', 'Lys-18' and 'Lys- 56' (H3K9ac, H3K18ac and H3K56ac, respectively), suppressing target gene expression of several transcription factors, including NF-kappa-B (PubMed:19625767, PubMed:21362626, PubMed:23892288, PubMed:23911928, PubMed:24012758, PubMed:26456828, PubMed:26898756, PubMed:27043296, PubMed:27180906, PubMed:30374165, PubMed:33067423). Acts as an inhibitor of transcription elongation by mediating deacetylation of H3K9ac and H3K56ac, preventing release of NELFE from chromatin and causing transcriptional pausing (By similarity). Involved in DNA repair by promoting double-strand break (DSB) repair: acts as a DSB sensor by recognizing and binding DSB sites, leading to (1) recruitment of DNA repair proteins, such as SMARCA5/SNF2H, and (2) deacetylation of histone H3K9ac and H3K56ac (PubMed:23911928, PubMed:31995034, PubMed:32538779). SIRT6 participation to DSB repair is probably involved in extension of life span (By similarity). Also promotes DNA repair by deacetylating non-histone proteins, such as DDB2 and p53/TP53 (PubMed:29474172, PubMed:32789493). Specifically deacetylates H3K18ac at pericentric heterochromatin, thereby maintaining pericentric heterochromatin silencing at centromeres and protecting against genomic instability and cellular senescence (PubMed:27043296). Involved in telomere maintenance by catalyzing deacetylation of histone H3 in telomeric chromatin, regulating telomere position effect and telomere movement in response to DNA damage (PubMed:18337721, PubMed:19625767, PubMed:21847107). Required for embryonic stem cell differentiation by mediating histone deacetylation of H3K9ac (PubMed:25915124, PubMed:29555651). Plays a major role in metabolism by regulating processes such as glycolysis, gluconeogenesis, insulin secretion and lipid metabolism (PubMed:24012758, PubMed:26787900). Inhibits glycolysis via histone deacetylase activity and by acting as a corepressor of the transcription factor HIF1A, thereby controlling the expression of multiple glycolytic genes (By similarity). Has tumor suppressor activity by repressing glycolysis, thereby inhibiting the Warburg effect (PubMed:23217706). Also regulates glycolysis and tumorigenesis by mediating deacetylation and nuclear export of non- histone proteins, such as isoform M2 of PKM (PKM2) (PubMed:26787900). Acts as a negative regulator of gluconeogenesis by mediating deacetylation of non-histone proteins, such as FOXO1 and KAT2A/GCN5 (PubMed:23142079, PubMed:25009184). Promotes beta-oxidation of fatty acids during fasting by catalyzing deacetylation of NCOA2, inducing coactivation of PPARA (By similarity). Acts as a regulator of lipid catabolism in brown adipocytes, both by catalyzing deacetylation of histones and non-histone proteins, such as FOXO1 (By similarity). Also acts as a regulator of circadian rhythms, both by regulating expression of clock-controlled genes involved in lipid and carbohydrate metabolism, and by catalyzing deacetylation of PER2 (By similarity). The defatty-acylase activity is specifically involved in regulation of protein secretion (PubMed:23552949, PubMed:24052263, PubMed:27322069, PubMed:28406396). Has high activity toward long-chain fatty acyl groups and mediates protein-lysine demyristoylation and depalmitoylation of target proteins, such as RRAS2 and TNF, thereby regulating their secretion (PubMed:23552949, PubMed:28406396). Also acts as a mono-ADP- ribosyltransferase by mediating mono-ADP-ribosylation of PARP1, TRIM28/KAP1 or SMARCC2/BAF170 (PubMed:21680843, PubMed:22753495, PubMed:27322069, PubMed:27568560). Mono-ADP-ribosyltransferase activity is involved in DNA repair, cellular senescence, repression of LINE-1 retrotransposon elements and regulation of transcription (PubMed:21680843, PubMed:22753495, PubMed:27568560). |
| Cellular Location | Nucleus. Chromosome. Chromosome, telomere. Endoplasmic reticulum. Note=Predominantly nuclear (PubMed:18337721). Associated with pericentric heterochromatin and telomeric heterochromatin regions (PubMed:18337721, PubMed:27043296) Localizes to DNA damage sites: directly recognizes and binds double- strand breaks (DSBs) sites via a tunnel-like structure that has high affinity for DSBs (PubMed:21680843, PubMed:23911928, PubMed:27568560, PubMed:31995034, PubMed:32538779). A fraction localizes to the endoplasmic reticulum (PubMed:23552949). |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
SIRT6 is also known as SIR2L6. This gene encodes a member of the sirtuin family of proteins, homologs to the yeast Sir2 protein. Members of the sirtuin family are characterized by a sirtuin core domain and grouped into four classes. The functions of human sirtuins have not yet been determined; however, yeast sirtuin proteins are known to regulate epigenetic gene silencing and suppress recombination of rDNA. Studies suggest that the human sirtuins may function as intracellular regulatory proteins with mono-ADP-ribosyltransferase activity. The protein encoded by this gene is included in class IV of the sirtuin family. Alternative splicing results in multiple transcript variants.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.